| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405970 | Journal of Molecular Structure | 2013 | 11 Pages |
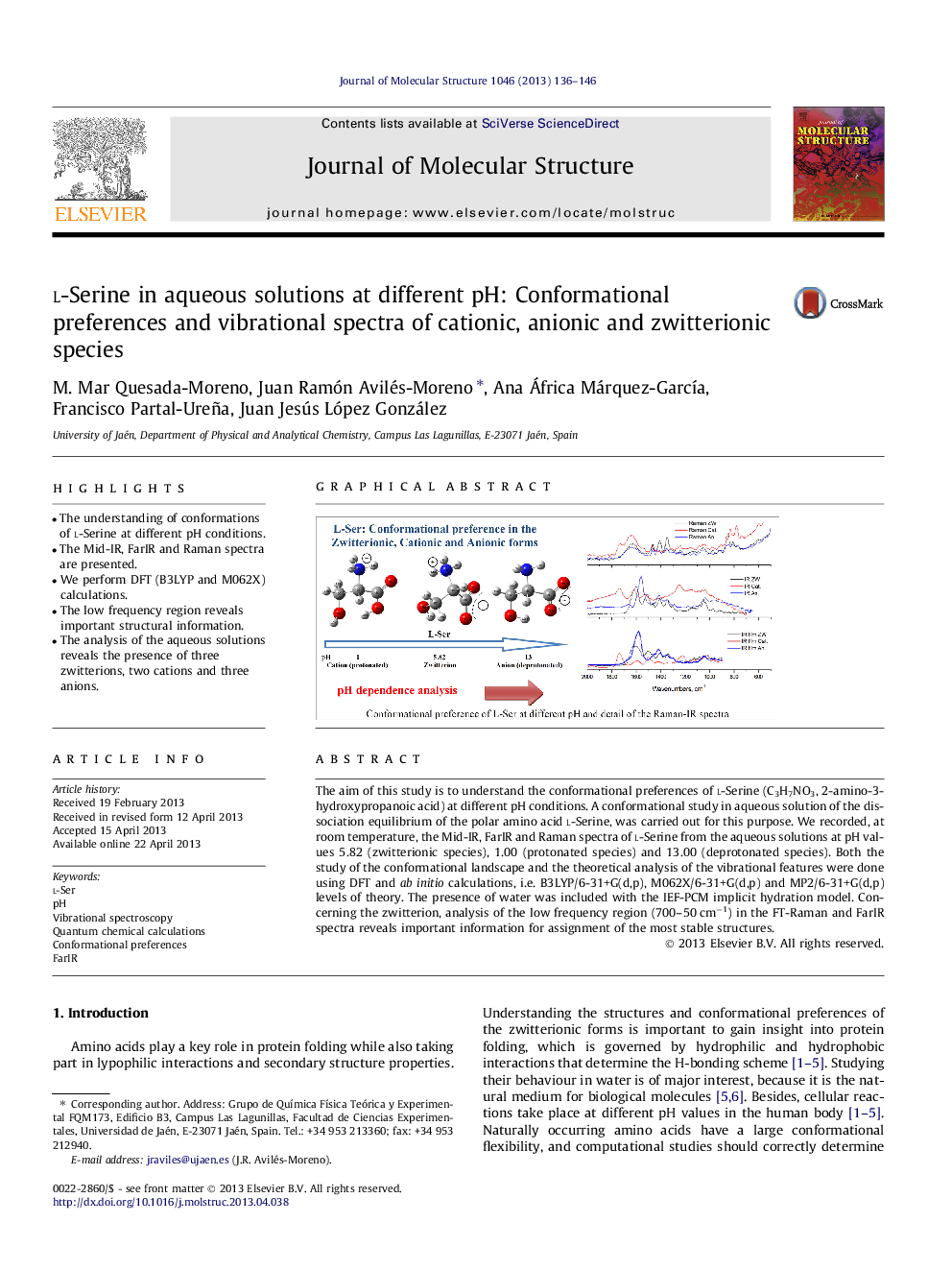
•The understanding of conformations of l-Serine at different pH conditions.•The Mid-IR, FarIR and Raman spectra are presented.•We perform DFT (B3LYP and M062X) calculations.•The low frequency region reveals important structural information.•The analysis of the aqueous solutions reveals the presence of three zwitterions, two cations and three anions.
The aim of this study is to understand the conformational preferences of l-Serine (C3H7NO3, 2-amino-3-hydroxypropanoic acid) at different pH conditions. A conformational study in aqueous solution of the dissociation equilibrium of the polar amino acid l-Serine, was carried out for this purpose. We recorded, at room temperature, the Mid-IR, FarIR and Raman spectra of l-Serine from the aqueous solutions at pH values 5.82 (zwitterionic species), 1.00 (protonated species) and 13.00 (deprotonated species). Both the study of the conformational landscape and the theoretical analysis of the vibrational features were done using DFT and ab initio calculations, i.e. B3LYP/6-31+G(d,p), M062X/6-31+G(d,p) and MP2/6-31+G(d,p) levels of theory. The presence of water was included with the IEF-PCM implicit hydration model. Concerning the zwitterion, analysis of the low frequency region (700–50 cm−1) in the FT-Raman and FarIR spectra reveals important information for assignment of the most stable structures.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide