| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408120 | Journal of Molecular Structure | 2015 | 14 Pages |
•Some divalent and trivalent complexes of nalidixic acid were discussed.•Structures of complexes were fully spectroscopic characterizations.•Nalidixic acid reacts as a bidentate ligand.•The gold(III) complex has nano-scale range.•Anticancer activity was checked.
This article describes the synthesis, characterization, computational and biological assessments of some divalent and trivalent metal (Ca(II), Fe(III), Pd(II) and Au(III)) complexes of nalidixic acid (nixH). The structures of these complexes were assigned using elemental analyses and spectral measurements e.g., IR, Raman, 1H NMR, 13C NMR and electronic techniques. These results indicated that, nalidixic acid reacts as a bidentate ligand bound to the metal ion through the oxygen atoms of carbonyl and carboxylate groups. The molar conductance measurements of the complexes in DMSO correspond to be non-electrolyte nature. Thus, these complexes may be formulated as [Ca(nix)(Cl)(H2O)3]. H2O, [Fe(nix)(Cl)2(H2O)2]·3H2O, [Pd(nix)(Cl)(H2O)] and [Au(nix)(Cl)2]. Base on the Coats–Redfern and Horowitz–Metzeger methods, the kinetic thermodynamic parameters (E∗, ΔS∗, ΔH∗ and ΔG∗) of the thermal decomposition reactions have been calculated from thermogravimetric curves of TG and DTG. The nano-scale range of the nalidixic acid complexes have been discussed using X-ray powder diffraction (XRD), scanning electron microscope (SEM) and transmission electron microscopy (TEM) analyzer. The computational studies for the synthesized complexes have been estimated.
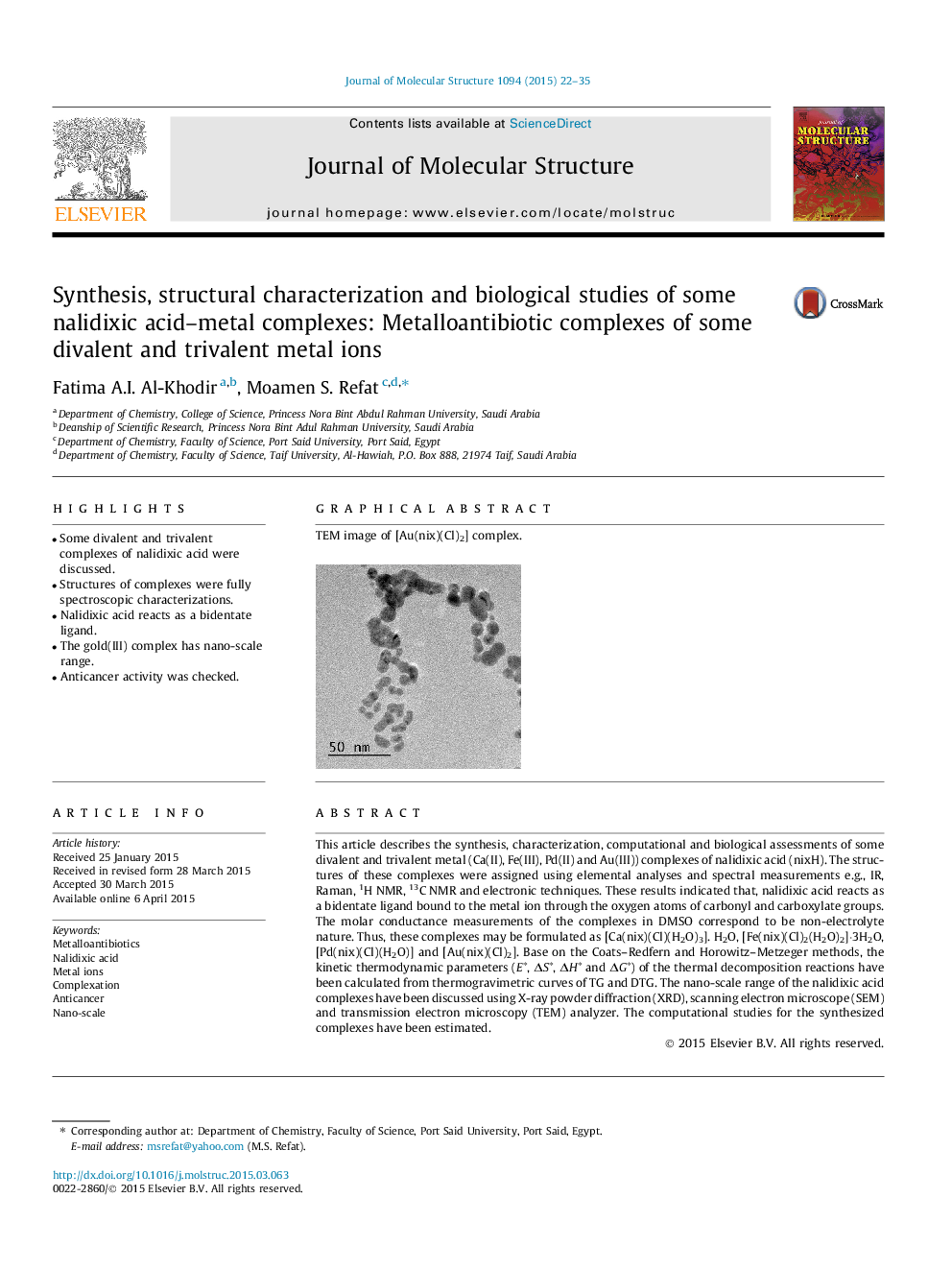
Graphical abstractTEM image of [Au(nix)(Cl)2] complex.Figure optionsDownload full-size imageDownload as PowerPoint slide