| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408142 | Journal of Molecular Structure | 2015 | 27 Pages |
•Spectroscopies properties were examined by FT-IR and FT-Raman spectra.•The complete vibrational assignments are made on the basis of Potential energy distribution.•NBO analysis and HOMO and LUMO energies are predicted.•Acid–base study was performed.
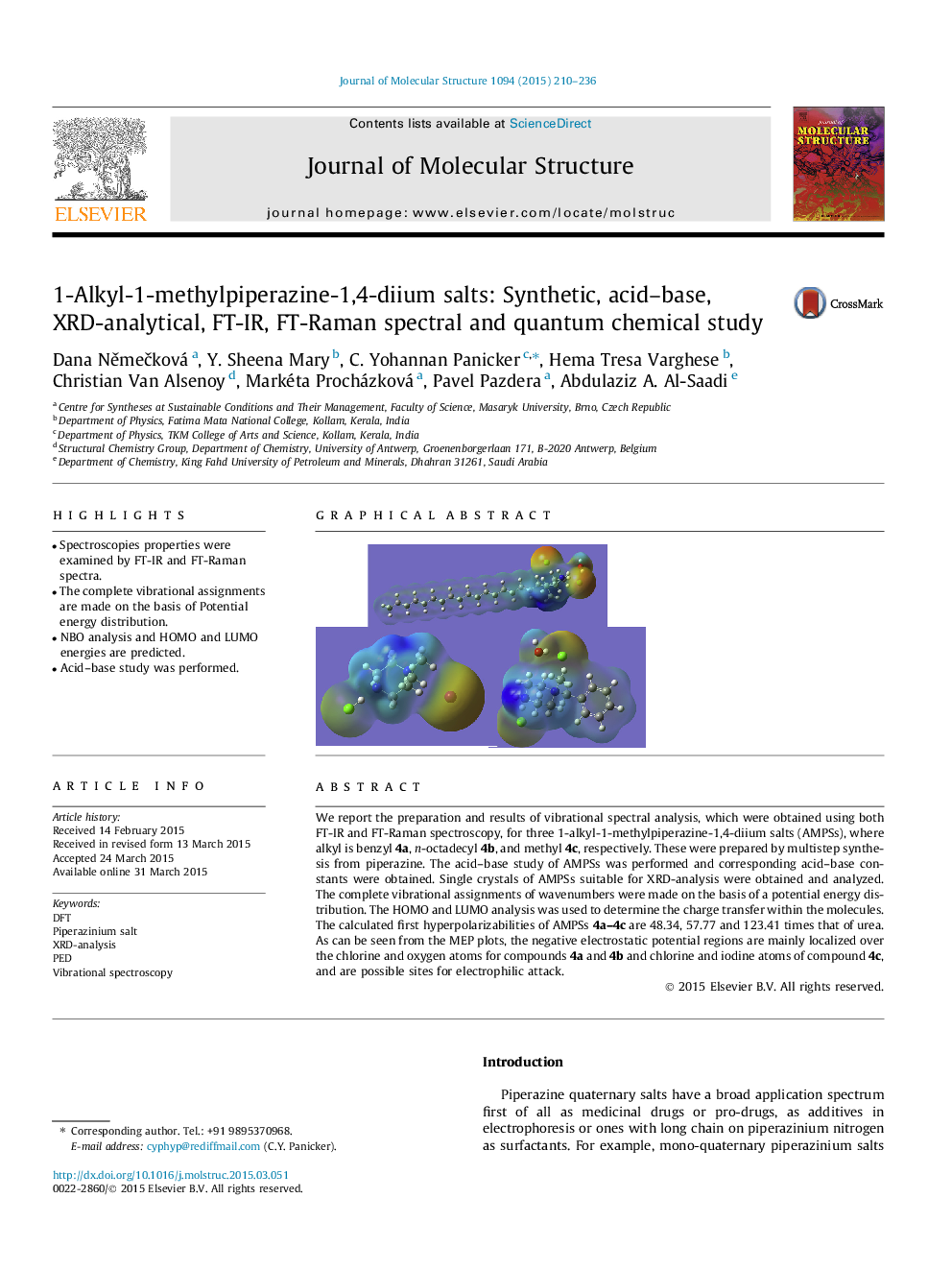
We report the preparation and results of vibrational spectral analysis, which were obtained using both FT-IR and FT-Raman spectroscopy, for three 1-alkyl-1-methylpiperazine-1,4-diium salts (AMPSs), where alkyl is benzyl 4a, n-octadecyl 4b, and methyl 4c, respectively. These were prepared by multistep synthesis from piperazine. The acid–base study of AMPSs was performed and corresponding acid–base constants were obtained. Single crystals of AMPSs suitable for XRD-analysis were obtained and analyzed. The complete vibrational assignments of wavenumbers were made on the basis of a potential energy distribution. The HOMO and LUMO analysis was used to determine the charge transfer within the molecules. The calculated first hyperpolarizabilities of AMPSs 4a–4c are 48.34, 57.77 and 123.41 times that of urea. As can be seen from the MEP plots, the negative electrostatic potential regions are mainly localized over the chlorine and oxygen atoms for compounds 4a and 4b and chlorine and iodine atoms of compound 4c, and are possible sites for electrophilic attack.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide