| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408301 | Journal of Molecular Structure | 2015 | 8 Pages |
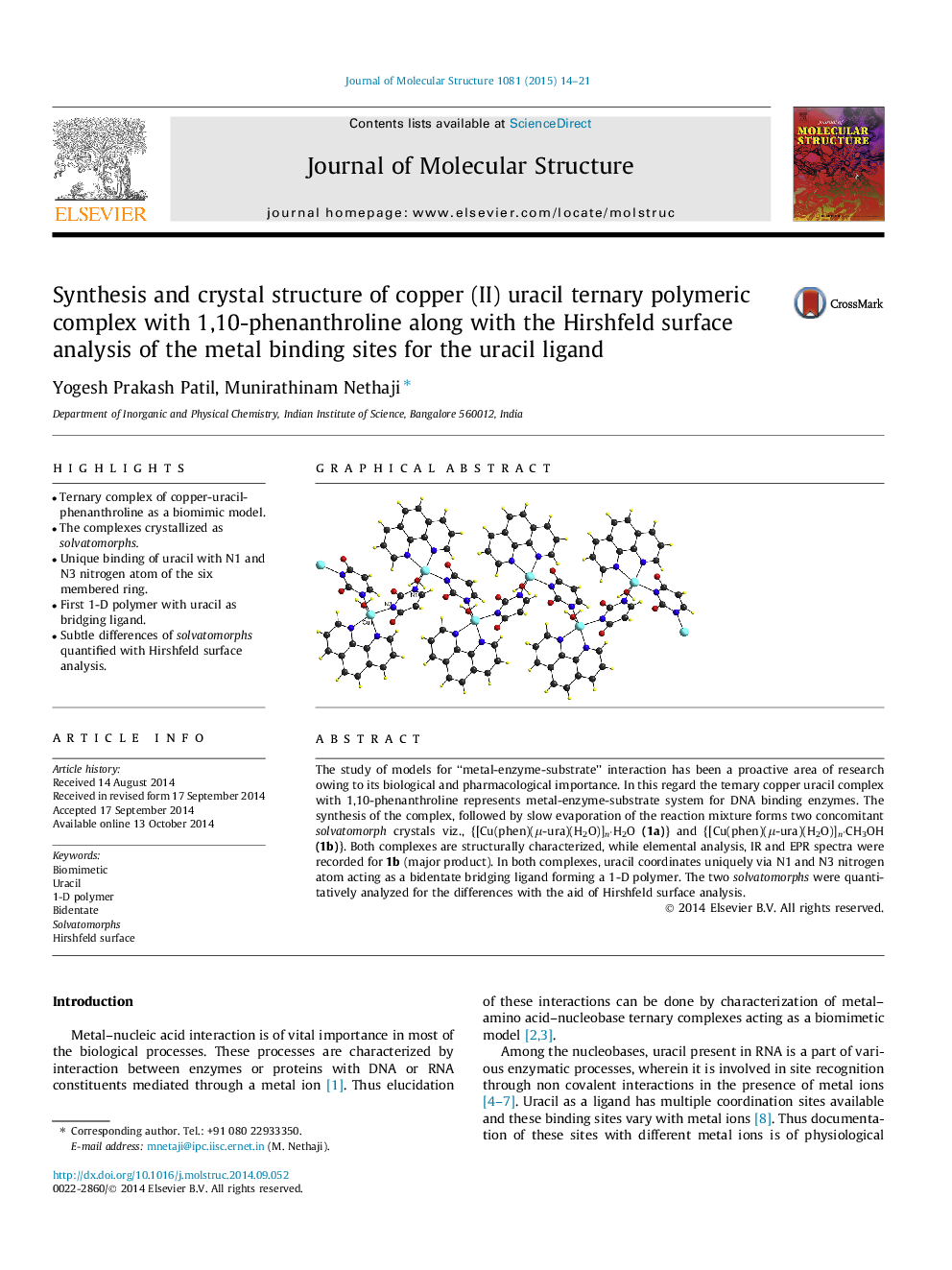
•Ternary complex of copper-uracil-phenanthroline as a biomimic model.•The complexes crystallized as solvatomorphs.•Unique binding of uracil with N1 and N3 nitrogen atom of the six membered ring.•First 1-D polymer with uracil as bridging ligand.•Subtle differences of solvatomorphs quantified with Hirshfeld surface analysis.
The study of models for “metal-enzyme-substrate” interaction has been a proactive area of research owing to its biological and pharmacological importance. In this regard the ternary copper uracil complex with 1,10-phenanthroline represents metal-enzyme-substrate system for DNA binding enzymes. The synthesis of the complex, followed by slow evaporation of the reaction mixture forms two concomitant solvatomorph crystals viz., {[Cu(phen)(μ-ura)(H2O)]n·H2O (1a)} and {[Cu(phen)(μ-ura)(H2O)]n·CH3OH (1b)}. Both complexes are structurally characterized, while elemental analysis, IR and EPR spectra were recorded for 1b (major product). In both complexes, uracil coordinates uniquely via N1 and N3 nitrogen atom acting as a bidentate bridging ligand forming a 1-D polymer. The two solvatomorphs were quantitatively analyzed for the differences with the aid of Hirshfeld surface analysis.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide