| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408323 | Journal of Molecular Structure | 2015 | 10 Pages |
•Synthesis of pyrazole appended quinoline chalcones.•Spectral, crystallographic and biological properties of chalcones.•E-configuration about the CC bond established via1H NMR & X-ray crystallography.•Antibacterial and antifungal studies showed promising activity of quinoline chalcones.
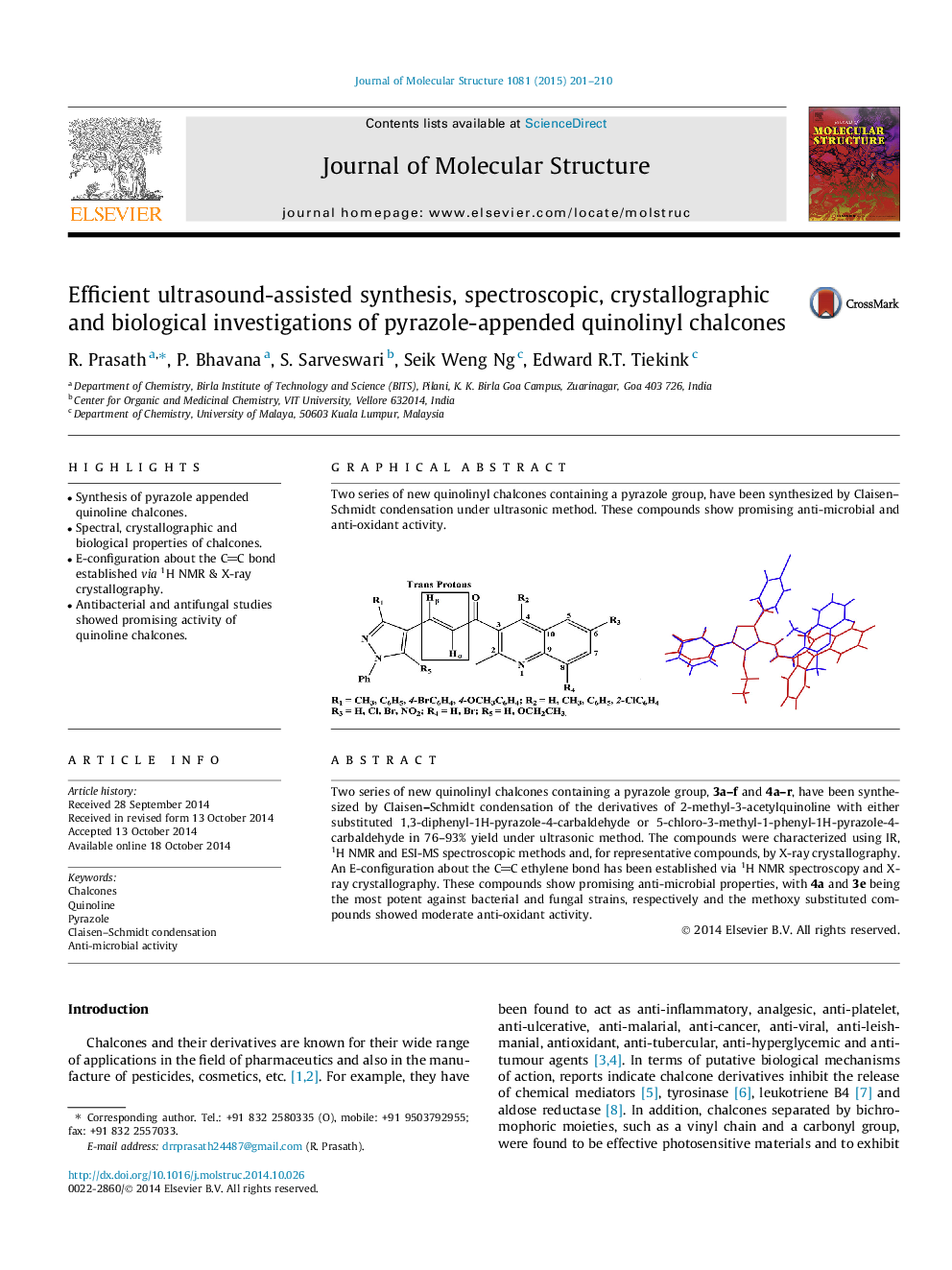
Two series of new quinolinyl chalcones containing a pyrazole group, 3a–f and 4a–r, have been synthesized by Claisen–Schmidt condensation of the derivatives of 2-methyl-3-acetylquinoline with either substituted 1,3-diphenyl-1H-pyrazole-4-carbaldehyde or 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde in 76–93% yield under ultrasonic method. The compounds were characterized using IR, 1H NMR and ESI-MS spectroscopic methods and, for representative compounds, by X-ray crystallography. An E-configuration about the CC ethylene bond has been established via 1H NMR spectroscopy and X-ray crystallography. These compounds show promising anti-microbial properties, with 4a and 3e being the most potent against bacterial and fungal strains, respectively and the methoxy substituted compounds showed moderate anti-oxidant activity.
Graphical abstractTwo series of new quinolinyl chalcones containing a pyrazole group, have been synthesized by Claisen–Schmidt condensation under ultrasonic method. These compounds show promising anti-microbial and anti-oxidant activity.Figure optionsDownload full-size imageDownload as PowerPoint slide