| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408328 | Journal of Molecular Structure | 2015 | 7 Pages |
•Co(III) and Zn(II) complexes with pyrazinamide synthesized.•The crystal structure of Co(III) complex has been determined.•The compounds show antibacterial activities.•Theoretical calculations of Co(III) complex are studied.
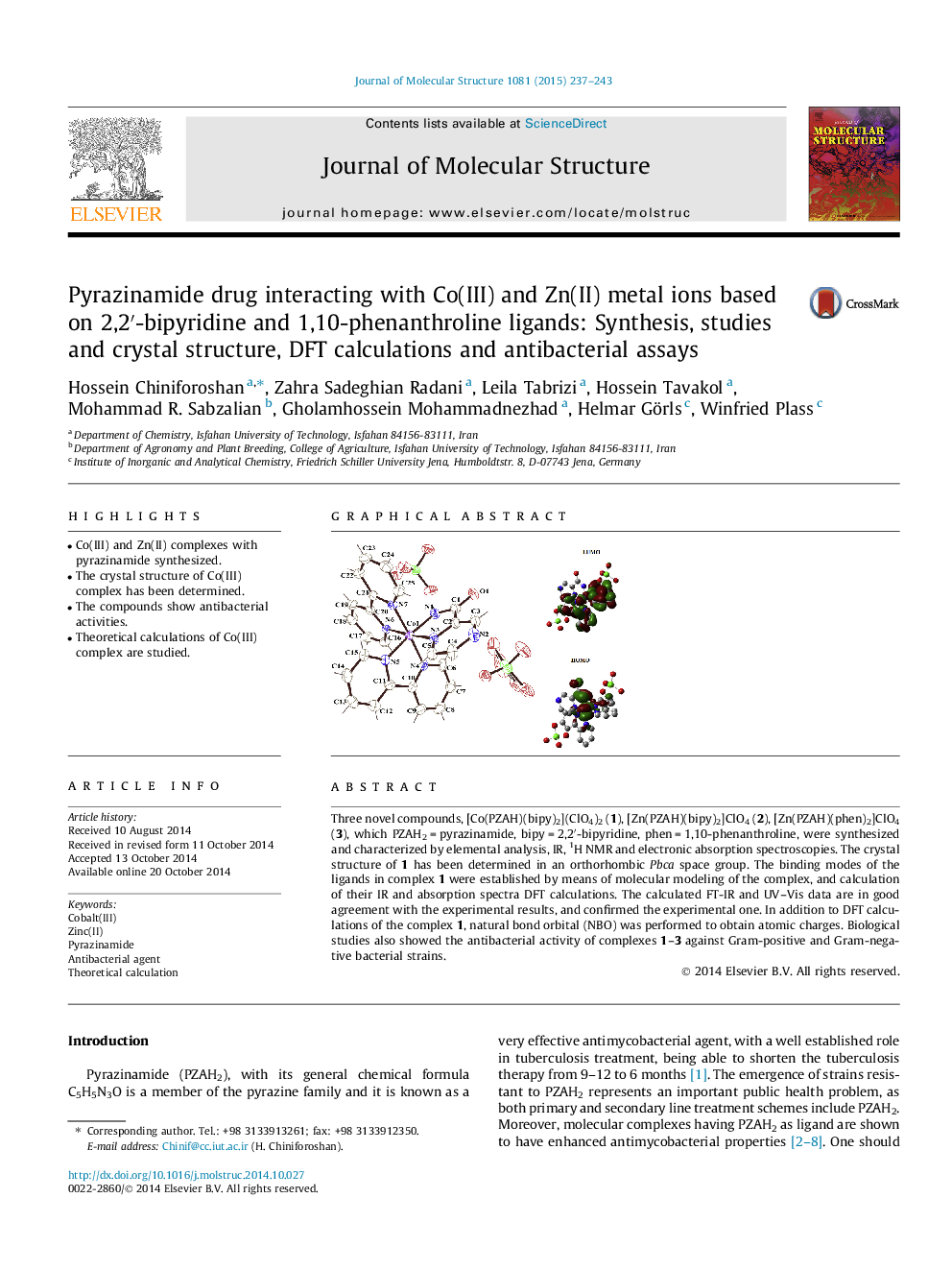
Three novel compounds, [Co(PZAH)(bipy)2](ClO4)2 (1), [Zn(PZAH)(bipy)2]ClO4 (2), [Zn(PZAH)(phen)2]ClO4 (3), which PZAH2 = pyrazinamide, bipy = 2,2′-bipyridine, phen = 1,10-phenanthroline, were synthesized and characterized by elemental analysis, IR, 1H NMR and electronic absorption spectroscopies. The crystal structure of 1 has been determined in an orthorhombic Pbca space group. The binding modes of the ligands in complex 1 were established by means of molecular modeling of the complex, and calculation of their IR and absorption spectra DFT calculations. The calculated FT-IR and UV–Vis data are in good agreement with the experimental results, and confirmed the experimental one. In addition to DFT calculations of the complex 1, natural bond orbital (NBO) was performed to obtain atomic charges. Biological studies also showed the antibacterial activity of complexes 1–3 against Gram-positive and Gram-negative bacterial strains.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide