| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408366 | Journal of Molecular Structure | 2015 | 13 Pages |
•The vibrational frequencies were calculated by DFT method.•FTIR and FT-Raman spectra of 2Br1HB were recorded.•NMR and UV–Vis spectra were also recorded and compared with calculated ones.•Molecular orbital contributions were studied by TDOS, PDOS and OPDOS.
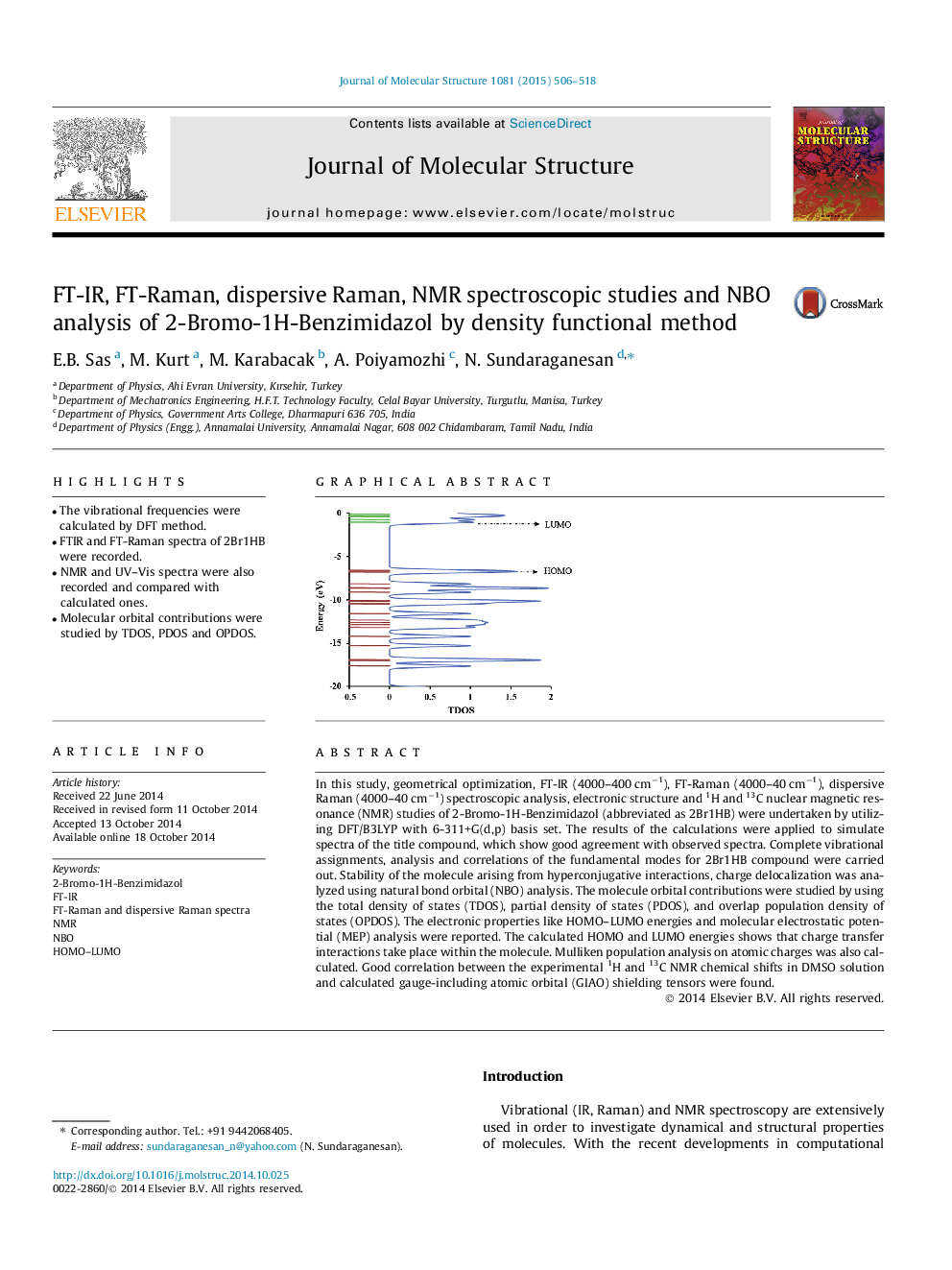
In this study, geometrical optimization, FT-IR (4000–400 cm−1), FT-Raman (4000–40 cm−1), dispersive Raman (4000–40 cm−1) spectroscopic analysis, electronic structure and 1H and 13C nuclear magnetic resonance (NMR) studies of 2-Bromo-1H-Benzimidazol (abbreviated as 2Br1HB) were undertaken by utilizing DFT/B3LYP with 6-311+G(d,p) basis set. The results of the calculations were applied to simulate spectra of the title compound, which show good agreement with observed spectra. Complete vibrational assignments, analysis and correlations of the fundamental modes for 2Br1HB compound were carried out. Stability of the molecule arising from hyperconjugative interactions, charge delocalization was analyzed using natural bond orbital (NBO) analysis. The molecule orbital contributions were studied by using the total density of states (TDOS), partial density of states (PDOS), and overlap population density of states (OPDOS). The electronic properties like HOMO–LUMO energies and molecular electrostatic potential (MEP) analysis were reported. The calculated HOMO and LUMO energies shows that charge transfer interactions take place within the molecule. Mulliken population analysis on atomic charges was also calculated. Good correlation between the experimental 1H and 13C NMR chemical shifts in DMSO solution and calculated gauge-including atomic orbital (GIAO) shielding tensors were found.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide