| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408434 | Journal of Molecular Structure | 2014 | 10 Pages |
•Single crystals of pseudomonic acid A were grown by slow solvent evaporation method.•The molecular structure was study by Hirshfeld surface and Monte Carlo conformational analysis.•Investigation by FT-IR, TG/DTG, 1H and 13C NMR methods.•Single-crystal X-ray study confirmed 3D hydrogen bond network.
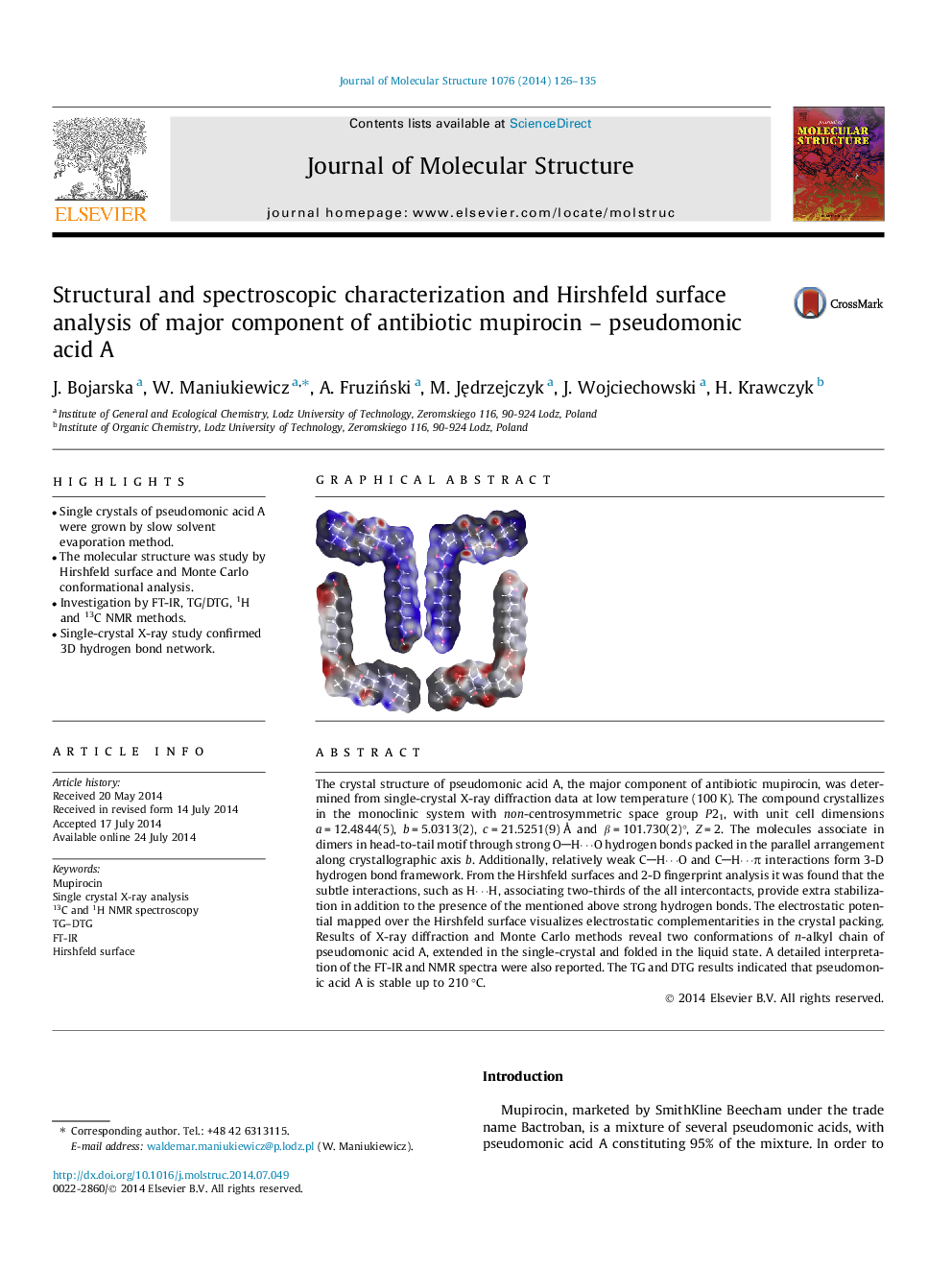
The crystal structure of pseudomonic acid A, the major component of antibiotic mupirocin, was determined from single-crystal X-ray diffraction data at low temperature (100 K). The compound crystallizes in the monoclinic system with non-centrosymmetric space group P21, with unit cell dimensions a = 12.4844(5), b = 5.0313(2), c = 21.5251(9) Å and β = 101.730(2)°, Z = 2. The molecules associate in dimers in head-to-tail motif through strong OH⋯O hydrogen bonds packed in the parallel arrangement along crystallographic axis b. Additionally, relatively weak CH⋯O and CH⋯π interactions form 3-D hydrogen bond framework. From the Hirshfeld surfaces and 2-D fingerprint analysis it was found that the subtle interactions, such as H⋯H, associating two-thirds of the all intercontacts, provide extra stabilization in addition to the presence of the mentioned above strong hydrogen bonds. The electrostatic potential mapped over the Hirshfeld surface visualizes electrostatic complementarities in the crystal packing. Results of X-ray diffraction and Monte Carlo methods reveal two conformations of n-alkyl chain of pseudomonic acid A, extended in the single-crystal and folded in the liquid state. A detailed interpretation of the FT-IR and NMR spectra were also reported. The TG and DTG results indicated that pseudomonic acid A is stable up to 210 °C.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide