| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408453 | Journal of Molecular Structure | 2014 | 6 Pages |
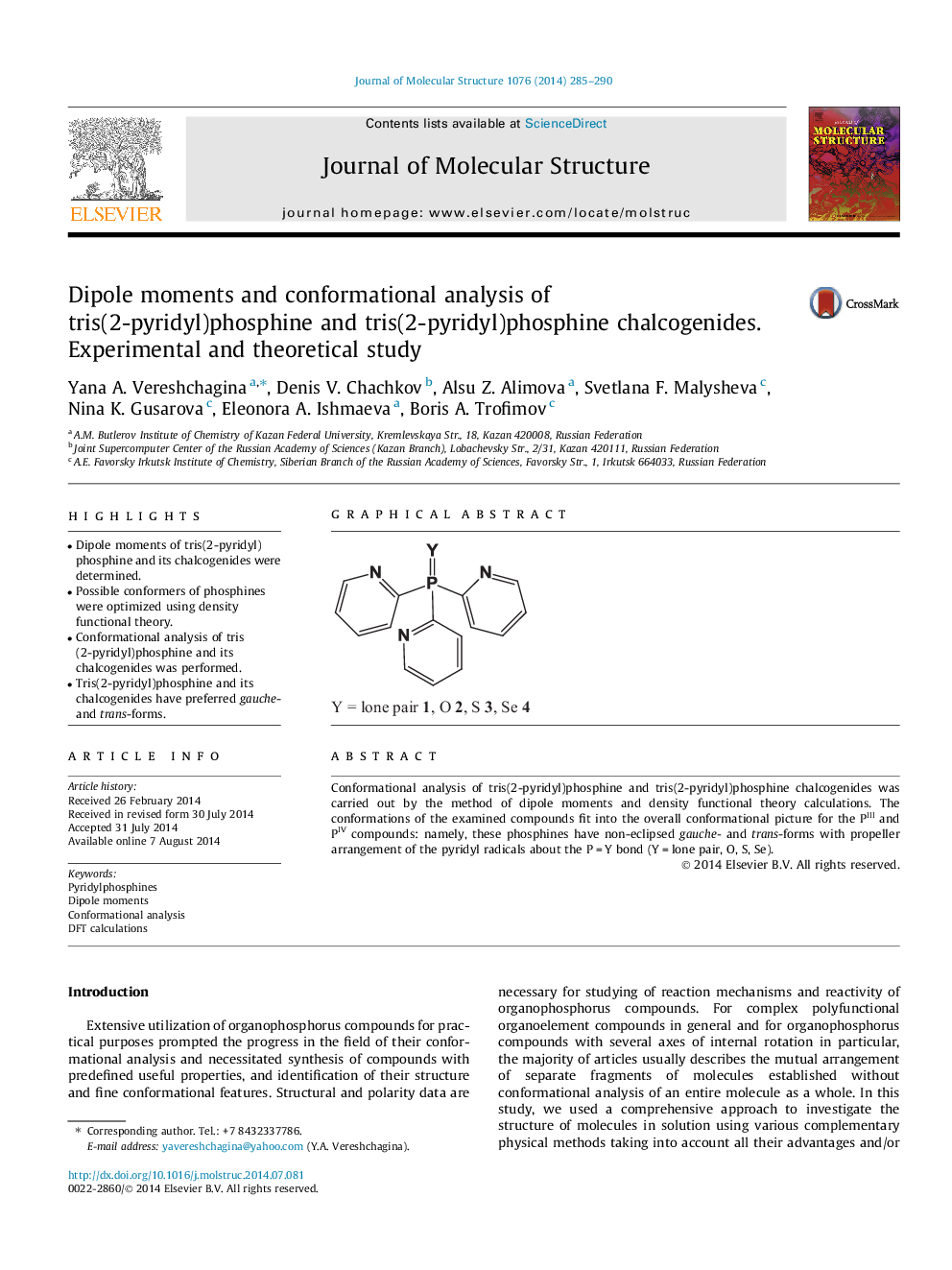
•Dipole moments of tris(2-pyridyl)phosphine and its chalcogenides were determined.•Possible conformers of phosphines were optimized using density functional theory.•Conformational analysis of tris(2-pyridyl)phosphine and its chalcogenides was performed.•Tris(2-pyridyl)phosphine and its chalcogenides have preferred gauche- and trans-forms.
Conformational analysis of tris(2-pyridyl)phosphine and tris(2-pyridyl)phosphine chalcogenides was carried out by the method of dipole moments and density functional theory calculations. The conformations of the examined compounds fit into the overall conformational picture for the PIII and PIV compounds: namely, these phosphines have non-eclipsed gauche- and trans-forms with propeller arrangement of the pyridyl radicals about the P = Y bond (Y = lone pair, O, S, Se).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide