| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408469 | Journal of Molecular Structure | 2014 | 8 Pages |
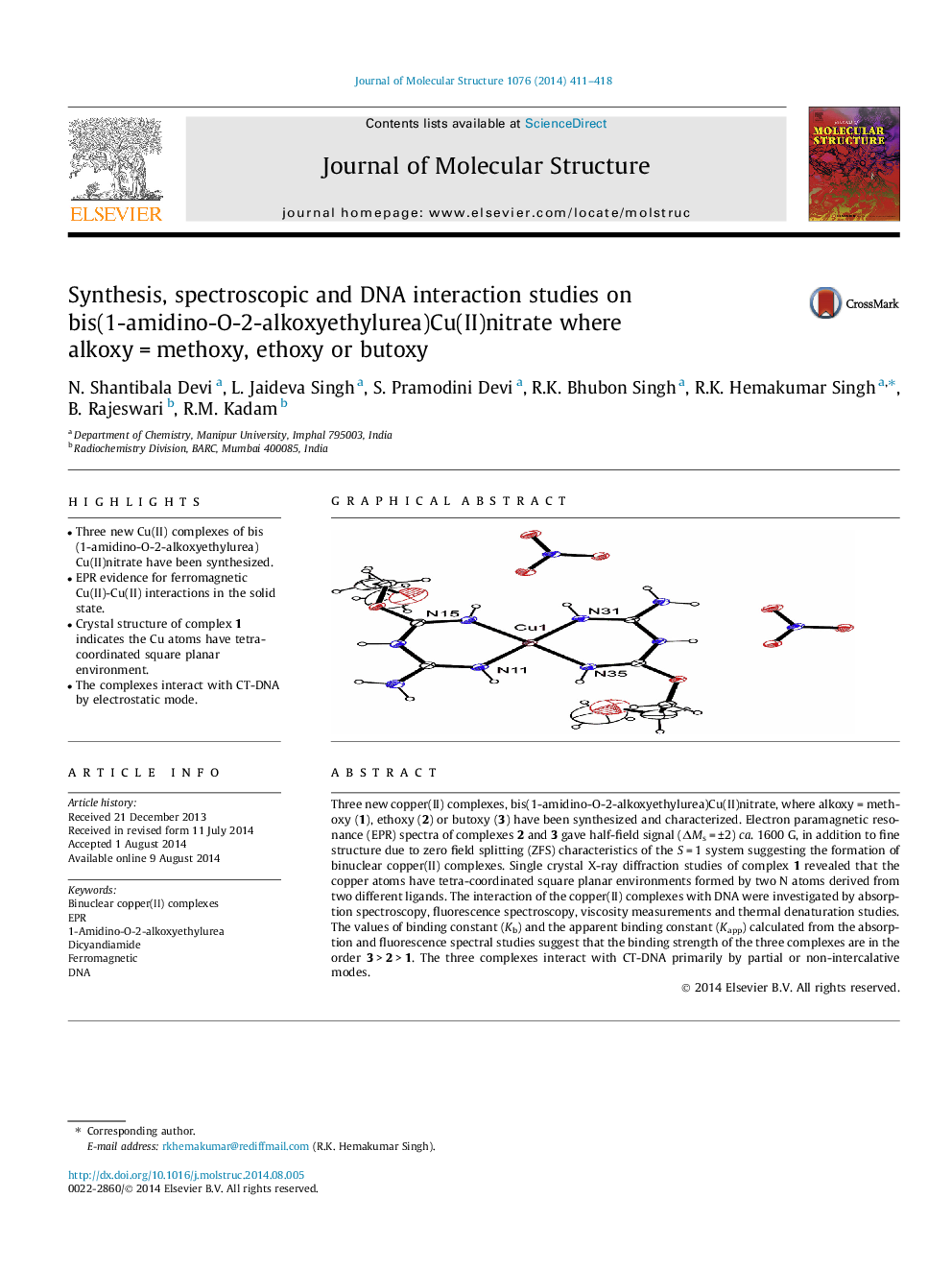
•Three new Cu(II) complexes of bis (1-amidino-O-2-alkoxyethylurea) Cu(II)nitrate have been synthesized.•EPR evidence for ferromagnetic Cu(II)-Cu(II) interactions in the solid state.•Crystal structure of complex 1 indicates the Cu atoms have tetra-coordinated square planar environment.•The complexes interact with CT-DNA by electrostatic mode.
Three new copper(II) complexes, bis(1-amidino-O-2-alkoxyethylurea)Cu(II)nitrate, where alkoxy = methoxy (1), ethoxy (2) or butoxy (3) have been synthesized and characterized. Electron paramagnetic resonance (EPR) spectra of complexes 2 and 3 gave half-field signal (ΔMs = ±2) ca. 1600 G, in addition to fine structure due to zero field splitting (ZFS) characteristics of the S = 1 system suggesting the formation of binuclear copper(II) complexes. Single crystal X-ray diffraction studies of complex 1 revealed that the copper atoms have tetra-coordinated square planar environments formed by two N atoms derived from two different ligands. The interaction of the copper(II) complexes with DNA were investigated by absorption spectroscopy, fluorescence spectroscopy, viscosity measurements and thermal denaturation studies. The values of binding constant (Kb) and the apparent binding constant (Kapp) calculated from the absorption and fluorescence spectral studies suggest that the binding strength of the three complexes are in the order 3 > 2 > 1. The three complexes interact with CT-DNA primarily by partial or non-intercalative modes.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide