| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408499 | Journal of Molecular Structure | 2014 | 10 Pages |
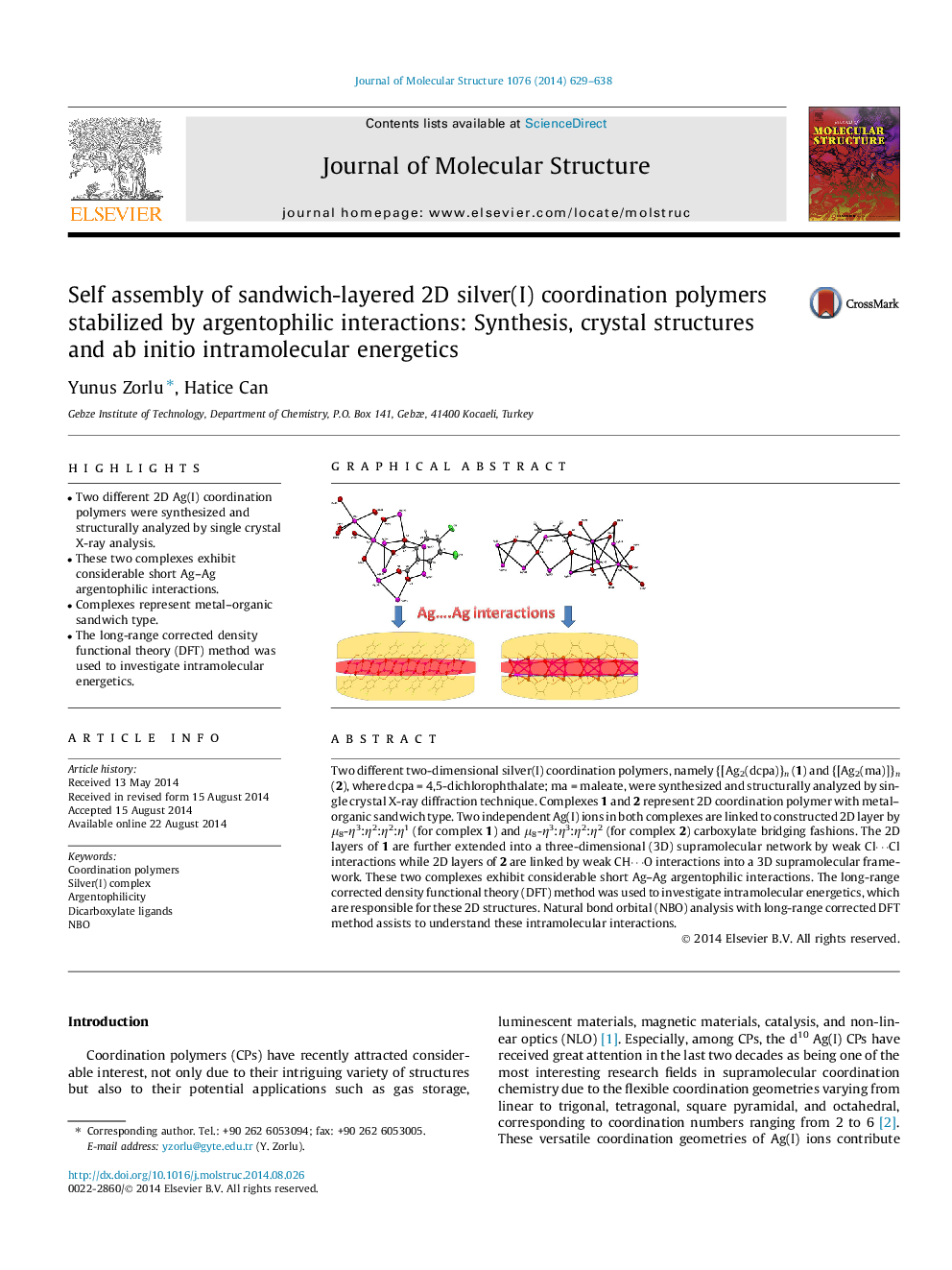
•Two different 2D Ag(I) coordination polymers were synthesized and structurally analyzed by single crystal X-ray analysis.•These two complexes exhibit considerable short Ag–Ag argentophilic interactions.•Complexes represent metal–organic sandwich type.•The long-range corrected density functional theory (DFT) method was used to investigate intramolecular energetics.
Two different two-dimensional silver(I) coordination polymers, namely {[Ag2(dcpa)}n (1) and {[Ag2(ma)]}n (2), where dcpa = 4,5-dichlorophthalate; ma = maleate, were synthesized and structurally analyzed by single crystal X-ray diffraction technique. Complexes 1 and 2 represent 2D coordination polymer with metal–organic sandwich type. Two independent Ag(I) ions in both complexes are linked to constructed 2D layer by μ8-η3:η2:η2:η1 (for complex 1) and μ8-η3:η3:η2:η2 (for complex 2) carboxylate bridging fashions. The 2D layers of 1 are further extended into a three-dimensional (3D) supramolecular network by weak Cl⋯Cl interactions while 2D layers of 2 are linked by weak CH⋯O interactions into a 3D supramolecular framework. These two complexes exhibit considerable short Ag–Ag argentophilic interactions. The long-range corrected density functional theory (DFT) method was used to investigate intramolecular energetics, which are responsible for these 2D structures. Natural bond orbital (NBO) analysis with long-range corrected DFT method assists to understand these intramolecular interactions.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide