| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408576 | Journal of Molecular Structure | 2014 | 9 Pages |
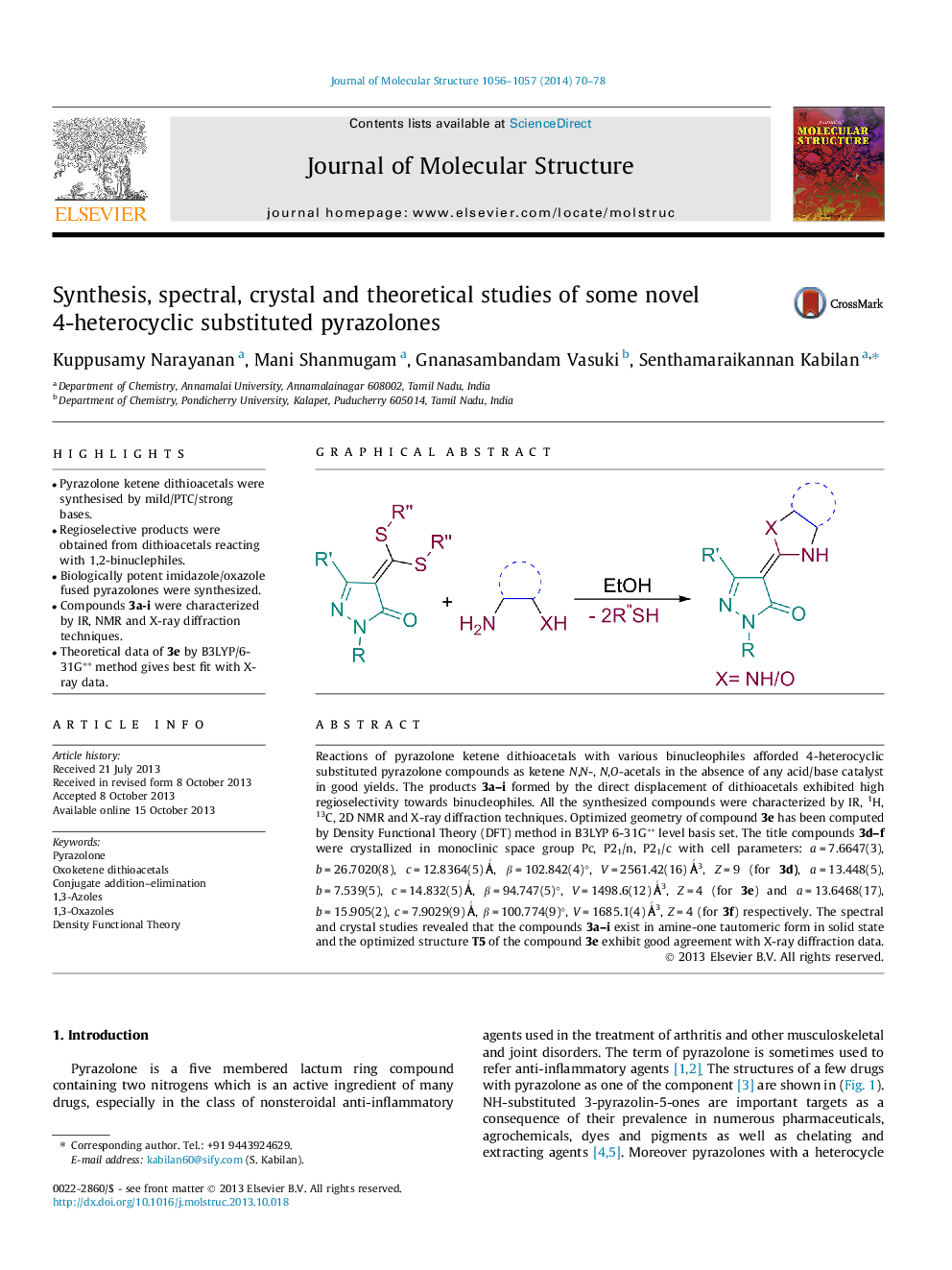
•Pyrazolone ketene dithioacetals were synthesised by mild/PTC/strong bases.•Regioselective products were obtained from dithioacetals reacting with 1,2-binuclephiles.•Biologically potent imidazole/oxazole fused pyrazolones were synthesized.•Compounds 3a-i were characterized by IR, NMR and X-ray diffraction techniques.•Theoretical data of 3e by B3LYP/6-31G⁎⁎ method gives best fit with X-ray data.
Reactions of pyrazolone ketene dithioacetals with various binucleophiles afforded 4-heterocyclic substituted pyrazolone compounds as ketene N,N-, N,O-acetals in the absence of any acid/base catalyst in good yields. The products 3a–i formed by the direct displacement of dithioacetals exhibited high regioselectivity towards binucleophiles. All the synthesized compounds were characterized by IR, 1H, 13C, 2D NMR and X-ray diffraction techniques. Optimized geometry of compound 3e has been computed by Density Functional Theory (DFT) method in B3LYP 6-31G∗∗ level basis set. The title compounds 3d–f were crystallized in monoclinic space group Pc, P21/n, P21/c with cell parameters: a = 7.6647(3), b = 26.7020(8), c = 12.8364(5) Ǻ, β = 102.842(4)°, V = 2561.42(16) Ǻ3, Z = 9 (for 3d), a = 13.448(5), b = 7.539(5), c = 14.832(5) Ǻ, β = 94.747(5)°, V = 1498.6(12) Ǻ3, Z = 4 (for 3e) and a = 13.6468(17), b = 15.905(2), c = 7.9029(9) Ǻ, β = 100.774(9)°, V = 1685.1(4) Ǻ3, Z = 4 (for 3f) respectively. The spectral and crystal studies revealed that the compounds 3a–i exist in amine-one tautomeric form in solid state and the optimized structure T5 of the compound 3e exhibit good agreement with X-ray diffraction data.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide