| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408610 | Journal of Molecular Structure | 2014 | 7 Pages |
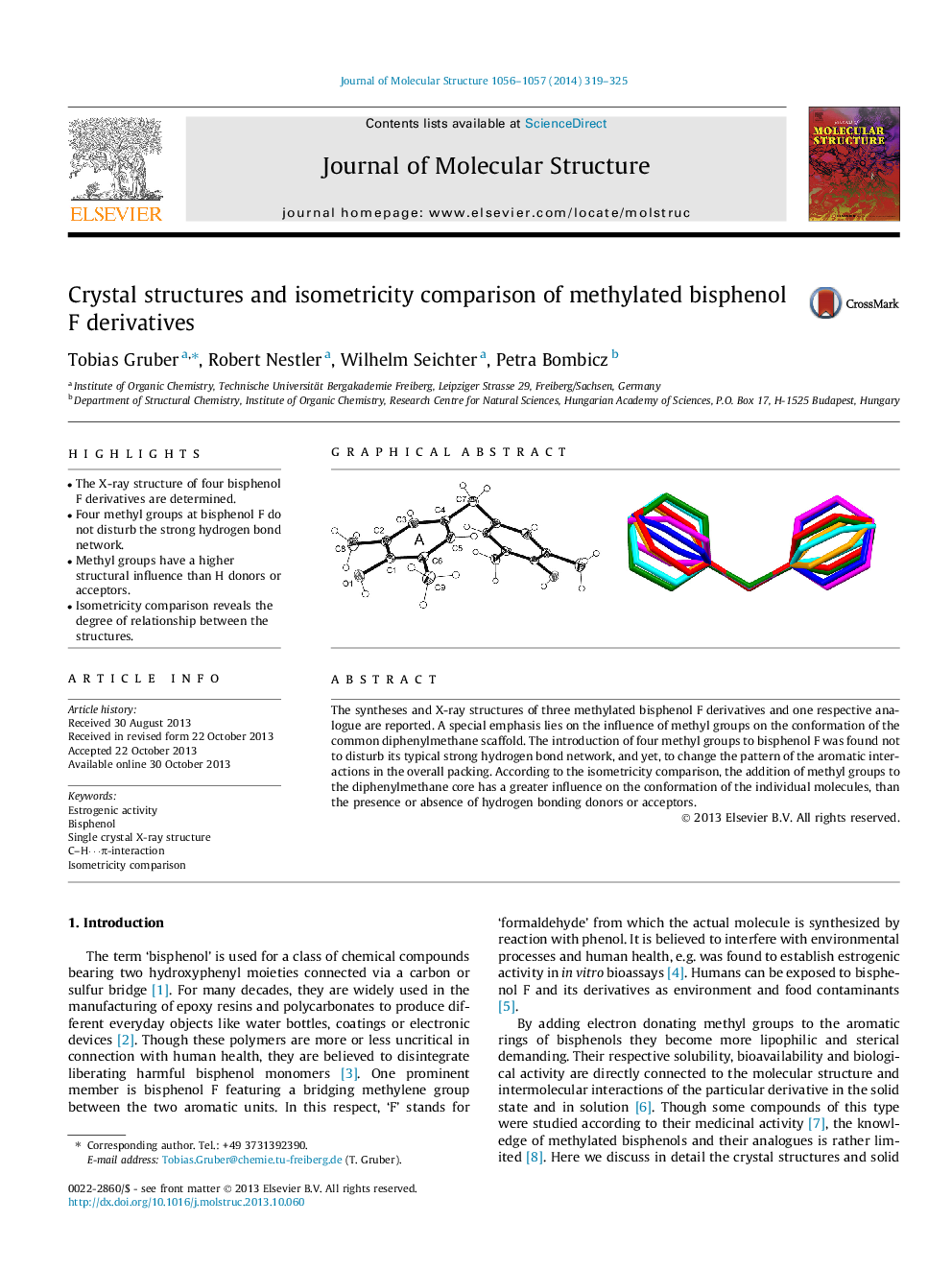
•The X-ray structure of four bisphenol F derivatives are determined.•Four methyl groups at bisphenol F do not disturb the strong hydrogen bond network.•Methyl groups have a higher structural influence than H donors or acceptors.•Isometricity comparison reveals the degree of relationship between the structures.
The syntheses and X-ray structures of three methylated bisphenol F derivatives and one respective analogue are reported. A special emphasis lies on the influence of methyl groups on the conformation of the common diphenylmethane scaffold. The introduction of four methyl groups to bisphenol F was found not to disturb its typical strong hydrogen bond network, and yet, to change the pattern of the aromatic interactions in the overall packing. According to the isometricity comparison, the addition of methyl groups to the diphenylmethane core has a greater influence on the conformation of the individual molecules, than the presence or absence of hydrogen bonding donors or acceptors.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide