| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1408861 | Journal of Molecular Structure | 2013 | 11 Pages |
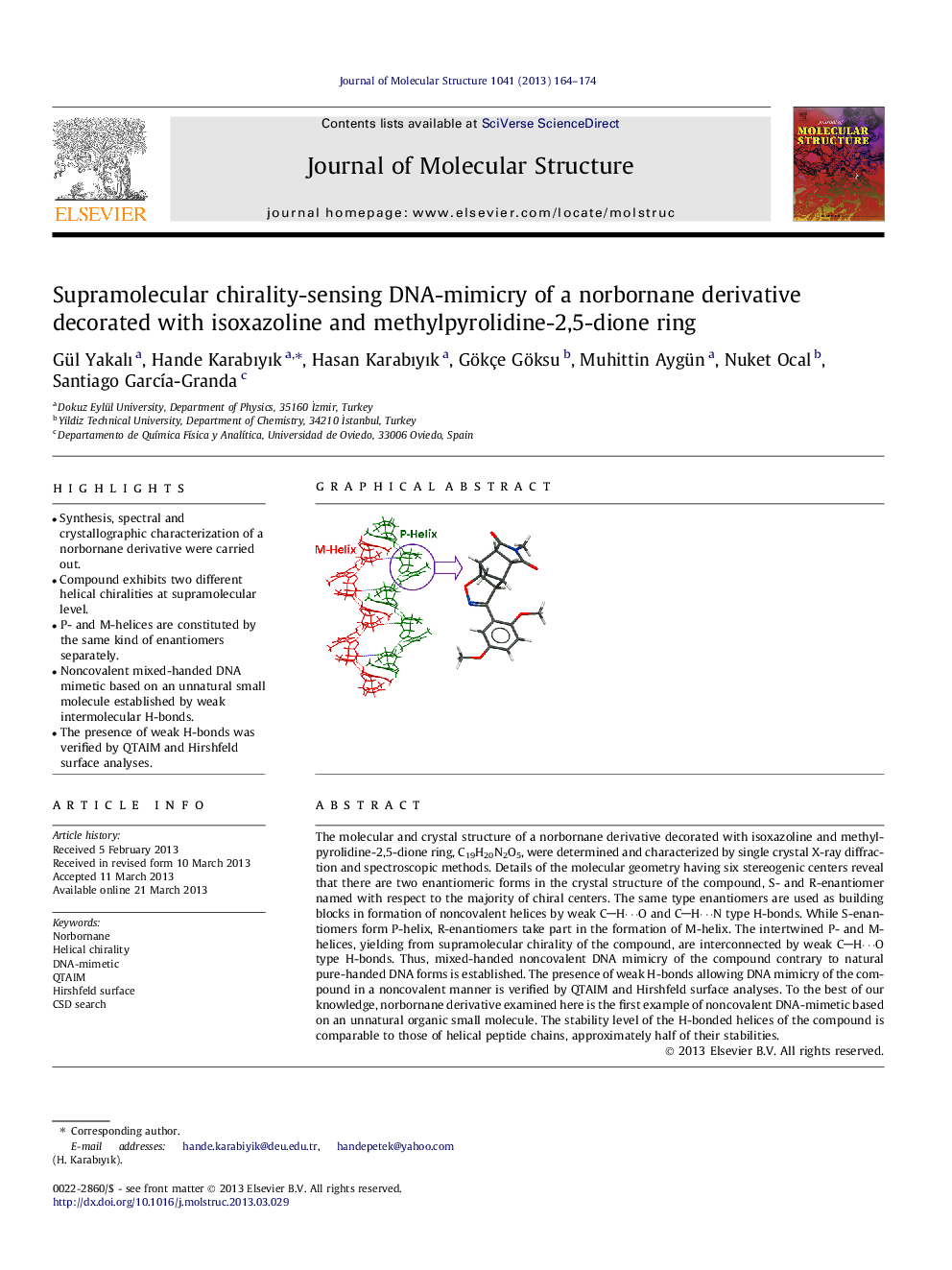
•Synthesis, spectral and crystallographic characterization of a norbornane derivative were carried out.•Compound exhibits two different helical chiralities at supramolecular level.•P- and M-helices are constituted by the same kind of enantiomers separately.•Noncovalent mixed-handed DNA mimetic based on an unnatural small molecule established by weak intermolecular H-bonds.•The presence of weak H-bonds was verified by QTAIM and Hirshfeld surface analyses.
The molecular and crystal structure of a norbornane derivative decorated with isoxazoline and methylpyrolidine-2,5-dione ring, C19H20N2O5, were determined and characterized by single crystal X-ray diffraction and spectroscopic methods. Details of the molecular geometry having six stereogenic centers reveal that there are two enantiomeric forms in the crystal structure of the compound, S- and R-enantiomer named with respect to the majority of chiral centers. The same type enantiomers are used as building blocks in formation of noncovalent helices by weak CH⋯O and CH⋯N type H-bonds. While S-enantiomers form P-helix, R-enantiomers take part in the formation of M-helix. The intertwined P- and M- helices, yielding from supramolecular chirality of the compound, are interconnected by weak CH⋯O type H-bonds. Thus, mixed-handed noncovalent DNA mimicry of the compound contrary to natural pure-handed DNA forms is established. The presence of weak H-bonds allowing DNA mimicry of the compound in a noncovalent manner is verified by QTAIM and Hirshfeld surface analyses. To the best of our knowledge, norbornane derivative examined here is the first example of noncovalent DNA-mimetic based on an unnatural organic small molecule. The stability level of the H-bonded helices of the compound is comparable to those of helical peptide chains, approximately half of their stabilities.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide