| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1409348 | Journal of Molecular Structure | 2014 | 10 Pages |
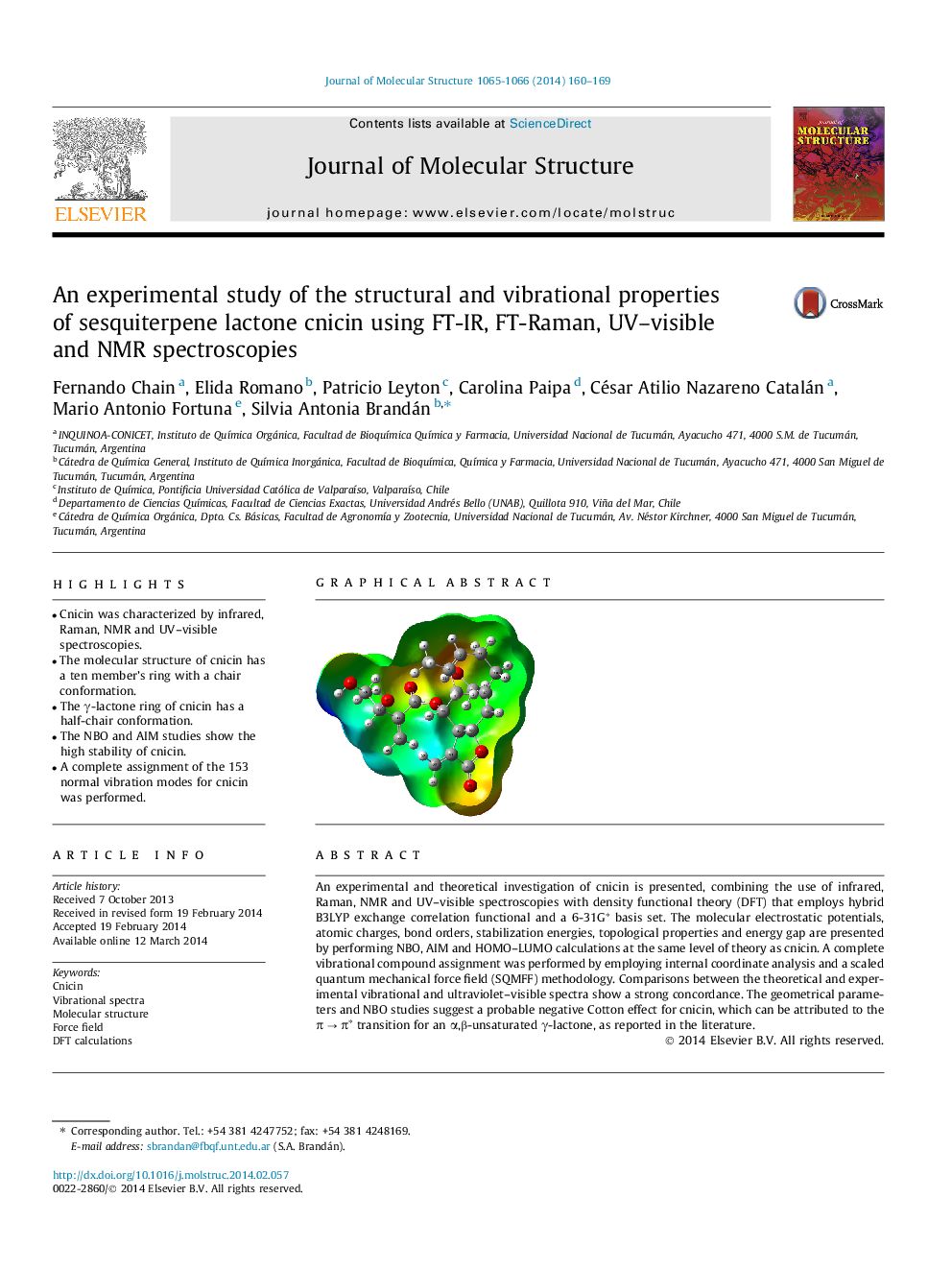
•Cnicin was characterized by infrared, Raman, NMR and UV–visible spectroscopies.•The molecular structure of cnicin has a ten member’s ring with a chair conformation.•The γ-lactone ring of cnicin has a half-chair conformation.•The NBO and AIM studies show the high stability of cnicin.•A complete assignment of the 153 normal vibration modes for cnicin was performed.
An experimental and theoretical investigation of cnicin is presented, combining the use of infrared, Raman, NMR and UV–visible spectroscopies with density functional theory (DFT) that employs hybrid B3LYP exchange correlation functional and a 6-31G∗ basis set. The molecular electrostatic potentials, atomic charges, bond orders, stabilization energies, topological properties and energy gap are presented by performing NBO, AIM and HOMO–LUMO calculations at the same level of theory as cnicin. A complete vibrational compound assignment was performed by employing internal coordinate analysis and a scaled quantum mechanical force field (SQMFF) methodology. Comparisons between the theoretical and experimental vibrational and ultraviolet–visible spectra show a strong concordance. The geometrical parameters and NBO studies suggest a probable negative Cotton effect for cnicin, which can be attributed to the π → π∗ transition for an α,β-unsaturated γ-lactone, as reported in the literature.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide