| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1886081 | Radiation Physics and Chemistry | 2014 | 7 Pages |
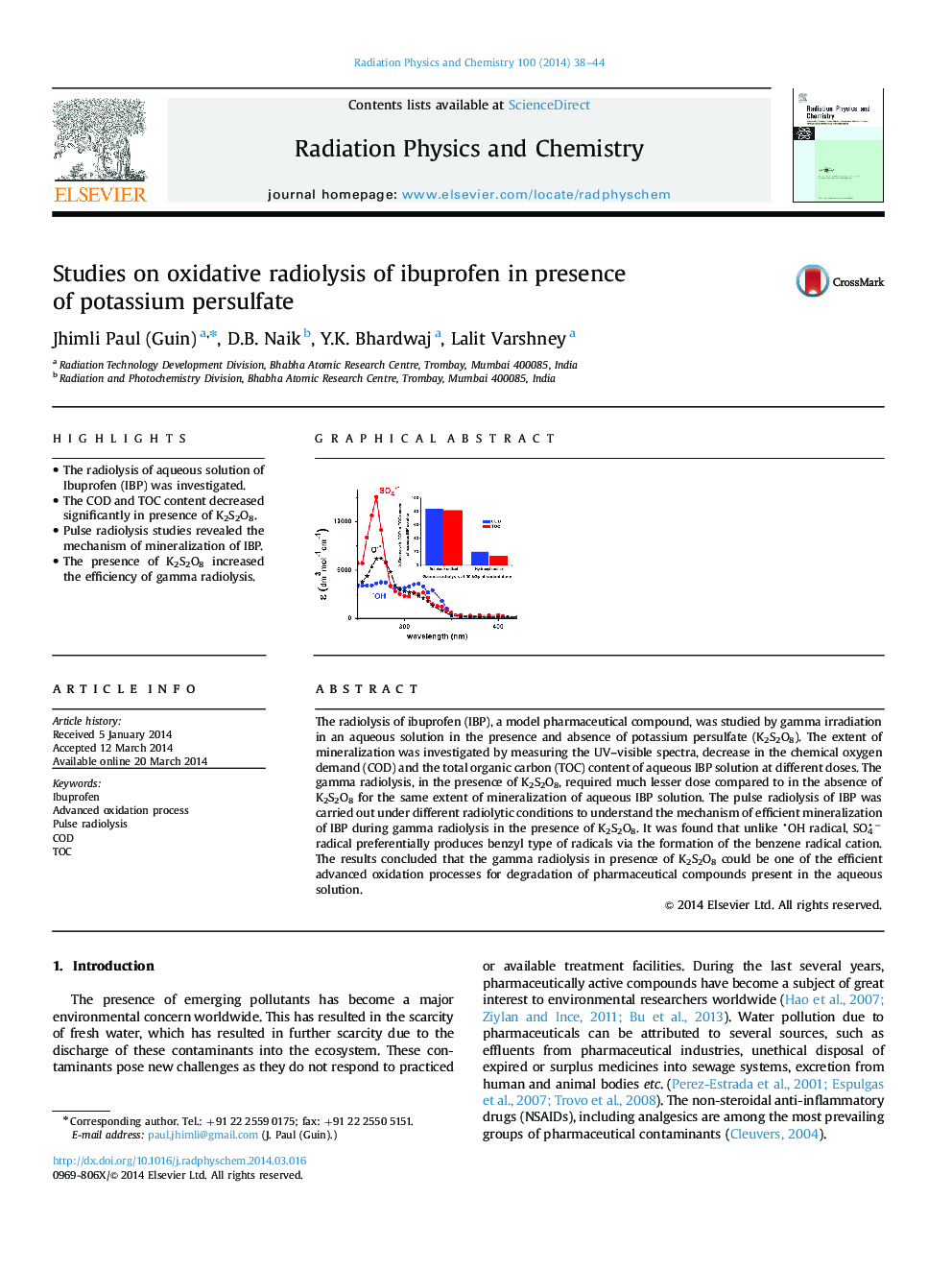
•The radiolysis of aqueous solution of Ibuprofen (IBP) was investigated.•The COD and TOC content decreased significantly in presence of K2S2O8.•Pulse radiolysis studies revealed the mechanism of mineralization of IBP.•The presence of K2S2O8 increased the efficiency of gamma radiolysis.
The radiolysis of ibuprofen (IBP), a model pharmaceutical compound, was studied by gamma irradiation in an aqueous solution in the presence and absence of potassium persulfate (K2S2O8). The extent of mineralization was investigated by measuring the UV–visible spectra, decrease in the chemical oxygen demand (COD) and the total organic carbon (TOC) content of aqueous IBP solution at different doses. The gamma radiolysis, in the presence of K2S2O8, required much lesser dose compared to in the absence of K2S2O8 for the same extent of mineralization of aqueous IBP solution. The pulse radiolysis of IBP was carried out under different radiolytic conditions to understand the mechanism of efficient mineralization of IBP during gamma radiolysis in the presence of K2S2O8. It was found that unlike OH radical, SO4− radical preferentially produces benzyl type of radicals via the formation of the benzene radical cation. The results concluded that the gamma radiolysis in presence of K2S2O8 could be one of the efficient advanced oxidation processes for degradation of pharmaceutical compounds present in the aqueous solution.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide