| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4360811 | Cell Host & Microbe | 2016 | 13 Pages |
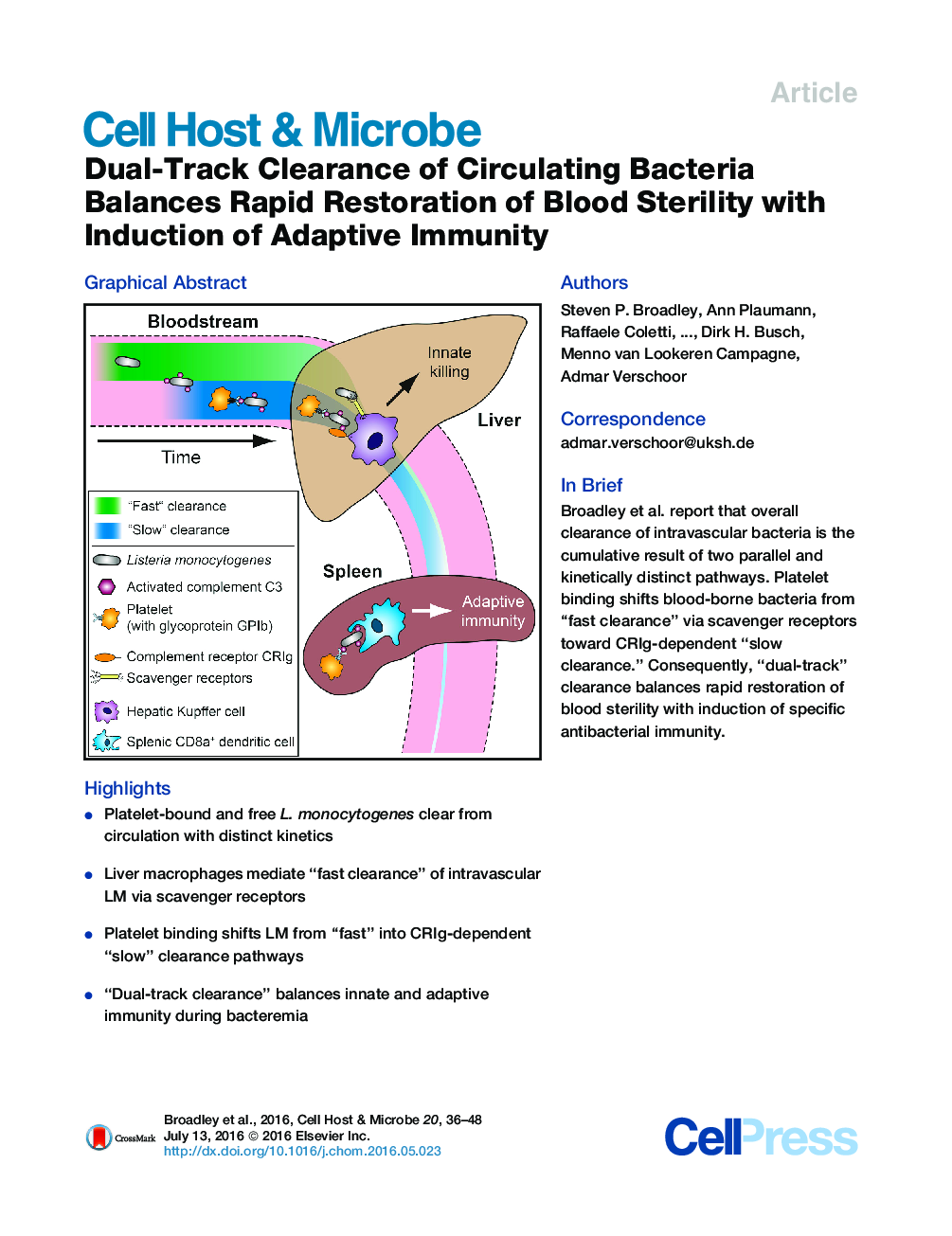
•Platelet-bound and free L. monocytogenes clear from circulation with distinct kinetics•Liver macrophages mediate “fast clearance” of intravascular LM via scavenger receptors•Platelet binding shifts LM from “fast” into CRIg-dependent “slow” clearance pathways•“Dual-track clearance” balances innate and adaptive immunity during bacteremia
SummaryEfficient clearance of bacteremia prevents life-threatening disease. Platelet binding to intravascular bacteria, a process involving platelet glycoprotein GPIb and bacterial opsonization with activated complement C3, influences blood clearance and anti-infective immunity. Using intravital microscopy of the bloodstream of mice infected with Listeria monocytogenes, we show that bacterial clearance is not a uniform process but a “dual-track” mechanism consisting of parallel “fast” and “slow” pathways. “Slow clearance” is regulated by time-dependent bacterial opsonization, stochastic platelet binding, and capture of bacteria-platelet-complexes via the complement receptor of the immunoglobulin superfamily, CRIg. The mechanism spares some bacteria from “fast clearance” and rapid destruction in the liver via Kupffer cell scavenger receptors, keeping them available for adaptive immunity induction by splenic CD8α+ dendritic cells. We consistently find “fast” and “slow” clearance patterns for a broad panel of other Gram+ and Gram− bacteria. Thus, dual-track clearance balances rapid restoration of blood sterility with induction of specific antibacterial immunity.
Graphical AbstractFigure optionsDownload full-size imageDownload high-quality image (225 K)Download as PowerPoint slide