| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4360850 | Cell Host & Microbe | 2016 | 14 Pages |
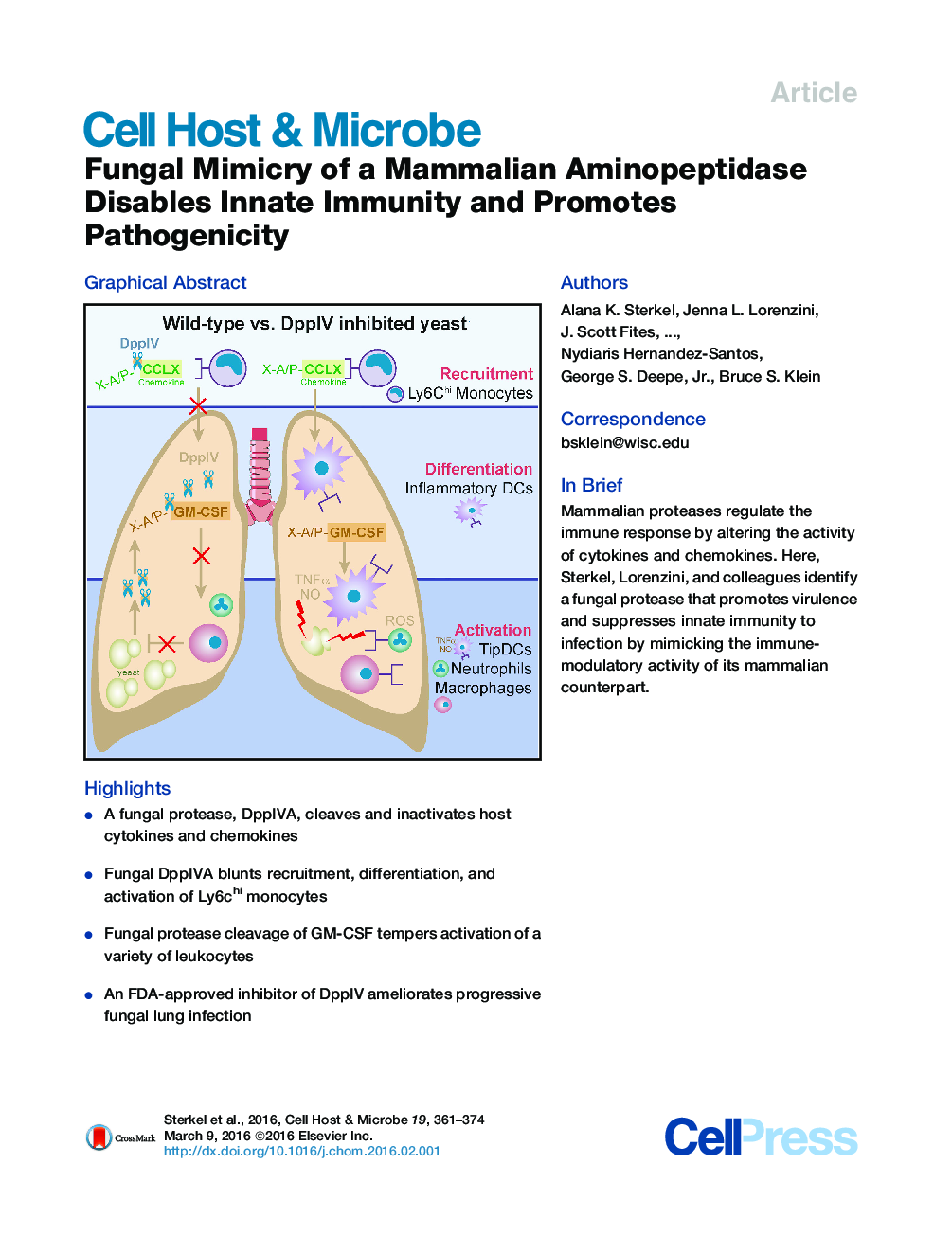
•A fungal protease, DppIVA, cleaves and inactivates host cytokines and chemokines•Fungal DppIVA blunts recruitment, differentiation, and activation of Ly6chi monocytes•Fungal protease cleavage of GM-CSF tempers activation of a variety of leukocytes•An FDA-approved inhibitor of DppIV ameliorates progressive fungal lung infection
SummarySystemic fungal infections trigger marked immune-regulatory disturbances, but the mechanisms are poorly understood. We report that the pathogenic yeast of Blastomyces dermatitidis elaborates dipeptidyl-peptidase IVA (DppIVA), a close mimic of the mammalian ectopeptidase CD26, which modulates critical aspects of hematopoiesis. We show that, like the mammalian enzyme, fungal DppIVA cleaved C-C chemokines and GM-CSF. Yeast producing DppIVA crippled the recruitment and differentiation of monocytes and prevented phagocyte activation and ROS production. Silencing fungal DppIVA gene expression curtailed virulence and restored recruitment of CCR2+ monocytes, generation of TipDC, and phagocyte killing of yeast. Pharmacological blockade of DppIVA restored leukocyte effector functions and stemmed infection, while addition of recombinant DppIVA to gene-silenced yeast enabled them to evade leukocyte defense. Thus, fungal DppIVA mediates immune-regulatory disturbances that underlie invasive fungal disease. These findings reveal a form of molecular piracy by a broadly conserved aminopeptidase during disease pathogenesis.
Graphical AbstractFigure optionsDownload full-size imageDownload high-quality image (236 K)Download as PowerPoint slide