| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4360882 | Cell Host & Microbe | 2015 | 10 Pages |
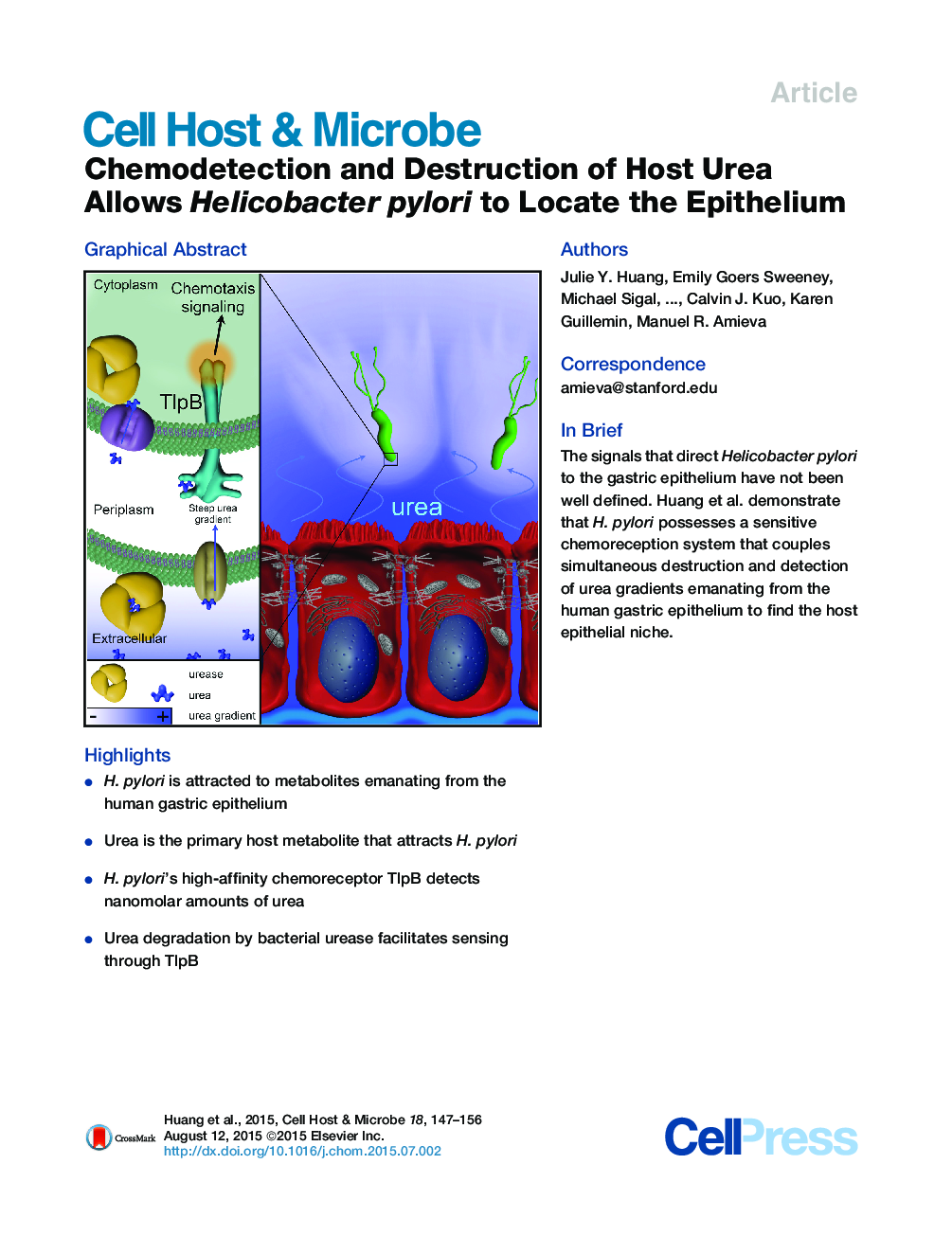
•H. pylori is attracted to metabolites emanating from the human gastric epithelium•Urea is the primary host metabolite that attracts H. pylori•H. pylori’s high-affinity chemoreceptor TlpB detects nanomolar amounts of urea•Urea degradation by bacterial urease facilitates sensing through TlpB
SummaryThe gastric pathogen Helicobacter pylori interacts intimately with the gastric mucosa to avoid the microbicidal acid in the stomach lumen. The cues H. pylori senses to locate and colonize the gastric epithelium have not been well defined. We show that metabolites emanating from human gastric organoids rapidly attract H. pylori. This response is largely controlled by the bacterial chemoreceptor TlpB, and the main attractant emanating from epithelia is urea. Our previous structural analyses show that TlpB binds urea with high affinity. Here we demonstrate that this tight binding controls highly sensitive responses, allowing detection of urea concentrations as low as 50 nM. Attraction to urea requires that H. pylori urease simultaneously destroys the signal. We propose that H. pylori has evolved a sensitive urea chemodetection and destruction system that allows the bacterium to dynamically and locally modify the host environment to locate the epithelium.
Graphical AbstractFigure optionsDownload full-size imageDownload high-quality image (292 K)Download as PowerPoint slide