| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4981848 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2017 | 9 Pages |
â¢PACl/(BS-12) complex sheds lights on its application in bubble functionalization.â¢Inorganic polymeric coagulant is effectively surface modified on microbubbles.â¢PACl concentration is key to stability of coagulative CGAs and nanosilica removal.â¢Flotation performs better at higher stability of coagulative CGAs.â¢Coagulant is dosed during bubble creation and no chemical-addition unit is needed.
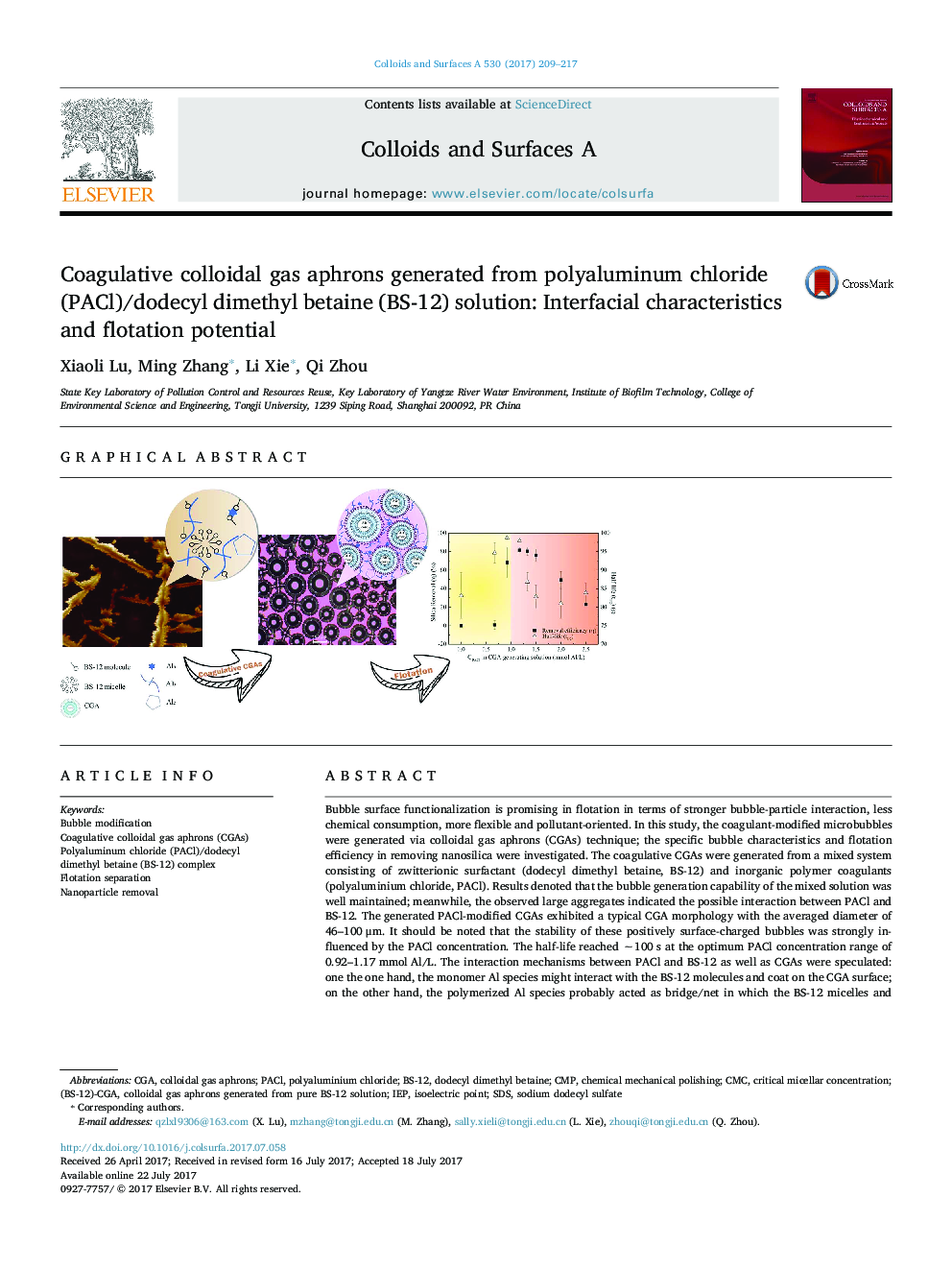
Bubble surface functionalization is promising in flotation in terms of stronger bubble-particle interaction, less chemical consumption, more flexible and pollutant-oriented. In this study, the coagulant-modified microbubbles were generated via colloidal gas aphrons (CGAs) technique; the specific bubble characteristics and flotation efficiency in removing nanosilica were investigated. The coagulative CGAs were generated from a mixed system consisting of zwitterionic surfactant (dodecyl dimethyl betaine, BS-12) and inorganic polymer coagulants (polyaluminium chloride, PACl). Results denoted that the bubble generation capability of the mixed solution was well maintained; meanwhile, the observed large aggregates indicated the possible interaction between PACl and BS-12. The generated PACl-modified CGAs exhibited a typical CGA morphology with the averaged diameter of 46-100 μm. It should be noted that the stability of these positively surface-charged bubbles was strongly influenced by the PACl concentration. The half-life reached â¼100 s at the optimum PACl concentration range of 0.92-1.17 mmol Al/L. The interaction mechanisms between PACl and BS-12 as well as CGAs were speculated: one the one hand, the monomer Al species might interact with the BS-12 molecules and coat on the CGA surface; on the other hand, the polymerized Al species probably acted as bridge/net in which the BS-12 micelles and CGAs were embedded. Particularly, the coagulative CGA-involved flotation could remove approximately 81% of nanosilica, and a reinforced flotation process was thus achieved.
Graphical abstractDownload high-res image (318KB)Download full-size image