| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4984295 | Journal of Colloid and Interface Science | 2018 | 10 Pages |
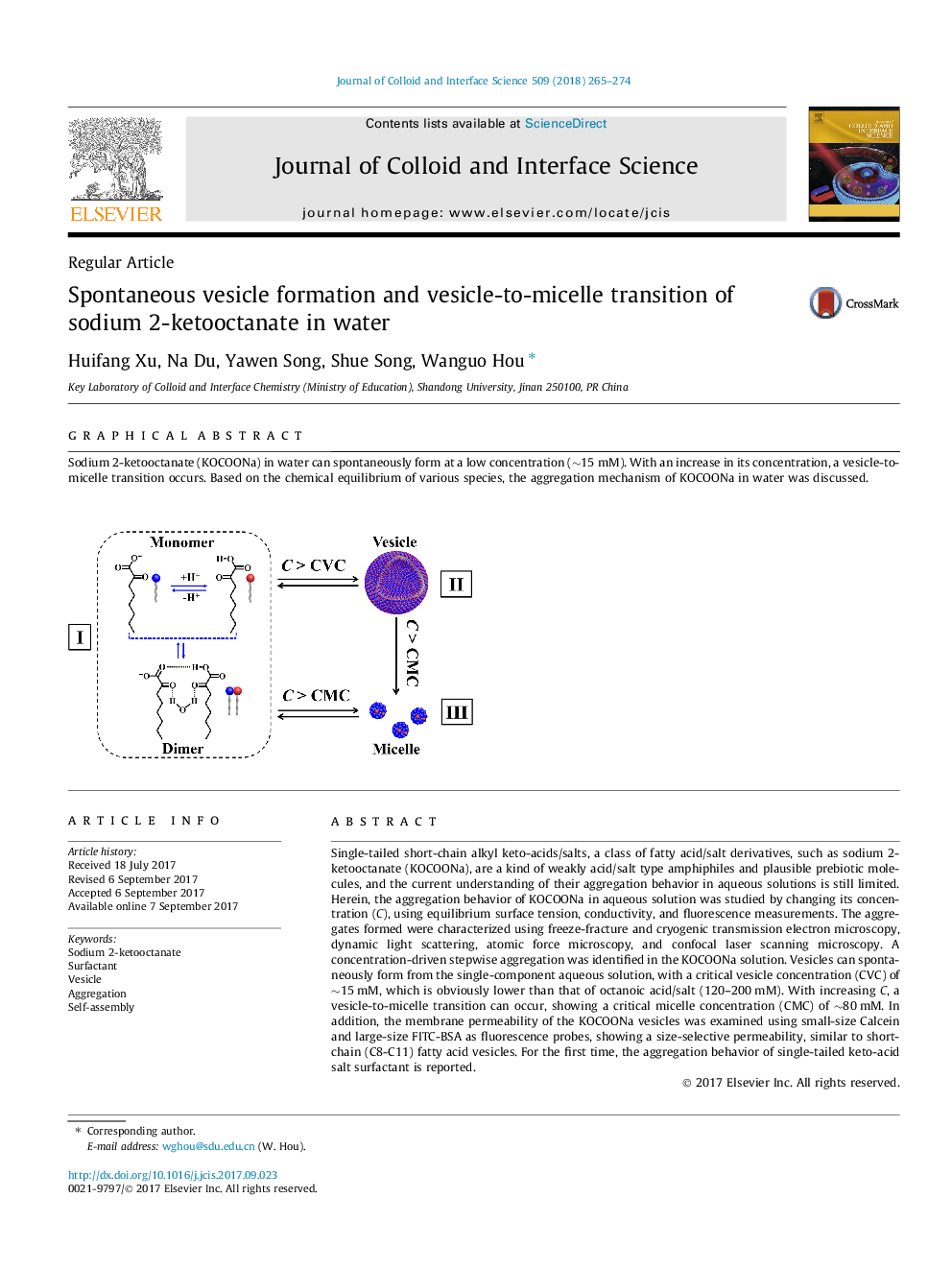
Single-tailed short-chain alkyl keto-acids/salts, a class of fatty acid/salt derivatives, such as sodium 2-ketooctanate (KOCOONa), are a kind of weakly acid/salt type amphiphiles and plausible prebiotic molecules, and the current understanding of their aggregation behavior in aqueous solutions is still limited. Herein, the aggregation behavior of KOCOONa in aqueous solution was studied by changing its concentration (C), using equilibrium surface tension, conductivity, and fluorescence measurements. The aggregates formed were characterized using freeze-fracture and cryogenic transmission electron microscopy, dynamic light scattering, atomic force microscopy, and confocal laser scanning microscopy. A concentration-driven stepwise aggregation was identified in the KOCOONa solution. Vesicles can spontaneously form from the single-component aqueous solution, with a critical vesicle concentration (CVC) of â¼15Â mM, which is obviously lower than that of octanoic acid/salt (120-200Â mM). With increasing C, a vesicle-to-micelle transition can occur, showing a critical micelle concentration (CMC) of â¼80Â mM. In addition, the membrane permeability of the KOCOONa vesicles was examined using small-size Calcein and large-size FITC-BSA as fluorescence probes, showing a size-selective permeability, similar to short-chain (C8-C11) fatty acid vesicles. For the first time, the aggregation behavior of single-tailed keto-acid salt surfactant is reported.
Graphical abstractSodium 2-ketooctanate (KOCOONa) in water can spontaneously form at a low concentration (â¼15Â mM). With an increase in its concentration, a vesicle-to-micelle transition occurs. Based on the chemical equilibrium of various species, the aggregation mechanism of KOCOONa in water was discussed.Download high-res image (149KB)Download full-size image