| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4984315 | Journal of Colloid and Interface Science | 2018 | 13 Pages |
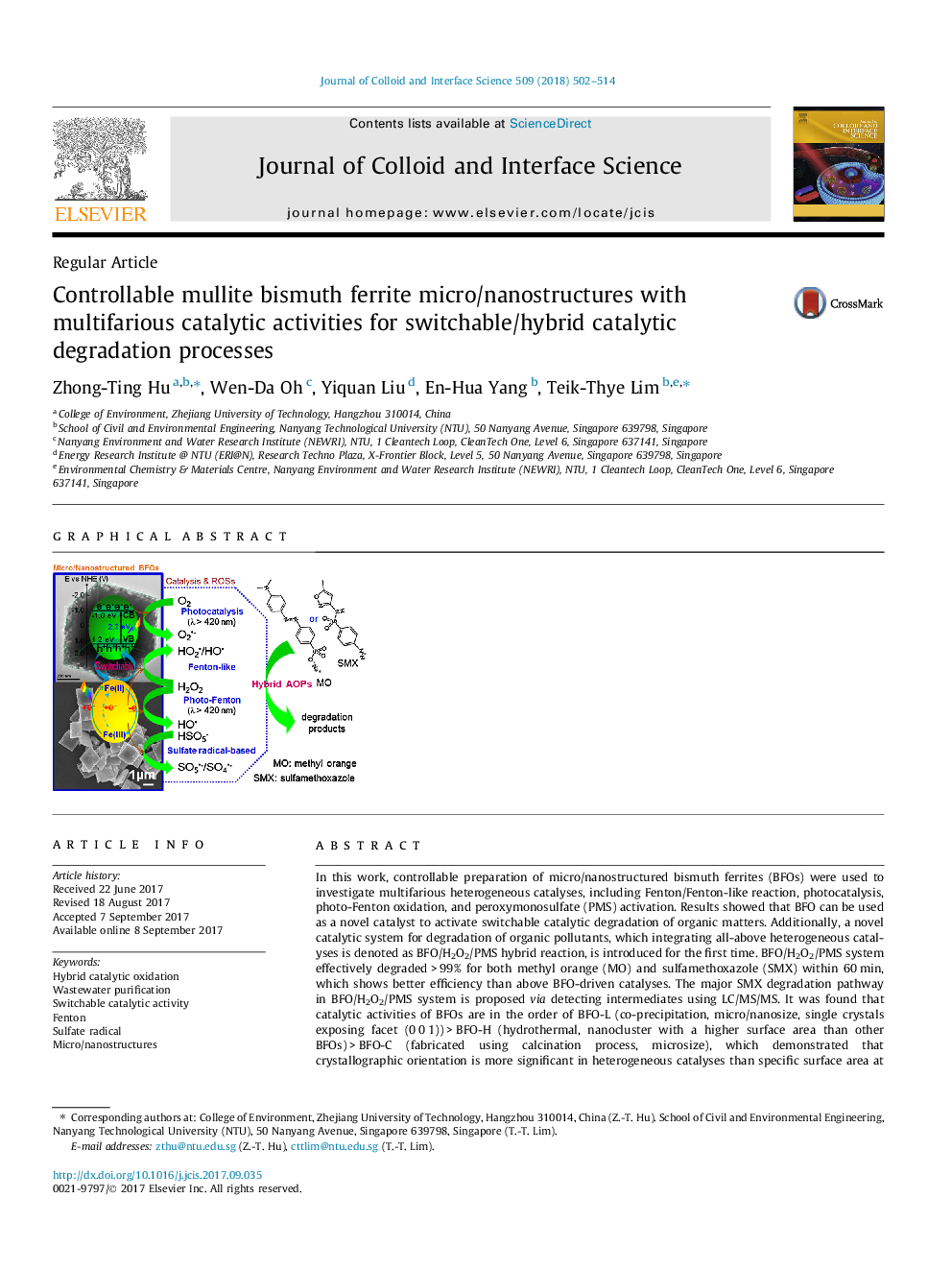
In this work, controllable preparation of micro/nanostructured bismuth ferrites (BFOs) were used to investigate multifarious heterogeneous catalyses, including Fenton/Fenton-like reaction, photocatalysis, photo-Fenton oxidation, and peroxymonosulfate (PMS) activation. Results showed that BFO can be used as a novel catalyst to activate switchable catalytic degradation of organic matters. Additionally, a novel catalytic system for degradation of organic pollutants, which integrating all-above heterogeneous catalyses is denoted as BFO/H2O2/PMS hybrid reaction, is introduced for the first time. BFO/H2O2/PMS system effectively degraded > 99% for both methyl orange (MO) and sulfamethoxazole (SMX) within 60 min, which shows better efficiency than above BFO-driven catalyses. The major SMX degradation pathway in BFO/H2O2/PMS system is proposed via detecting intermediates using LC/MS/MS. It was found that catalytic activities of BFOs are in the order of BFO-L (co-precipitation, micro/nanosize, single crystals exposing facet (0 0 1)) > BFO-H (hydrothermal, nanocluster with a higher surface area than other BFOs) > BFO-C (fabricated using calcination process, microsize), which demonstrated that crystallographic orientation is more significant in heterogeneous catalyses than specific surface area at micro/nanoscale. Besides, the required H2O2 consumption for achieving 99% TOC removal was identified in BFO-driven photo-Fenton oxidation. The other effects on degradation efficiency, such as H2O2 dosage and pH, were investigated as well. In Fenton/Fenton-like reaction, reaction conditions suggested are â¼61.5 mM H2O2 dosage and pH â¥Â 4.5 to avoid quenching of HO into HO2 by excessive H2O2 and Fe leaching.
Graphical abstractDownload high-res image (206KB)Download full-size image