| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5130606 | Analytica Chimica Acta | 2017 | 6 Pages |
â¢CdS QDs growing in aqueous buffered solutions are stabilized by cysteine (CSH).â¢Copper ions (Cu2+) modulate the growth of CSH-capped CdS QDs.â¢This system allows quantification of Cu2+ in real samples: bottled mineral and tap water.â¢Photoelectrochemical (PEC) analysis was performed using SPCEs modified with Os-PVP polymer.â¢PEC method for detection of Cu2+ ions outperforms the optical assays.
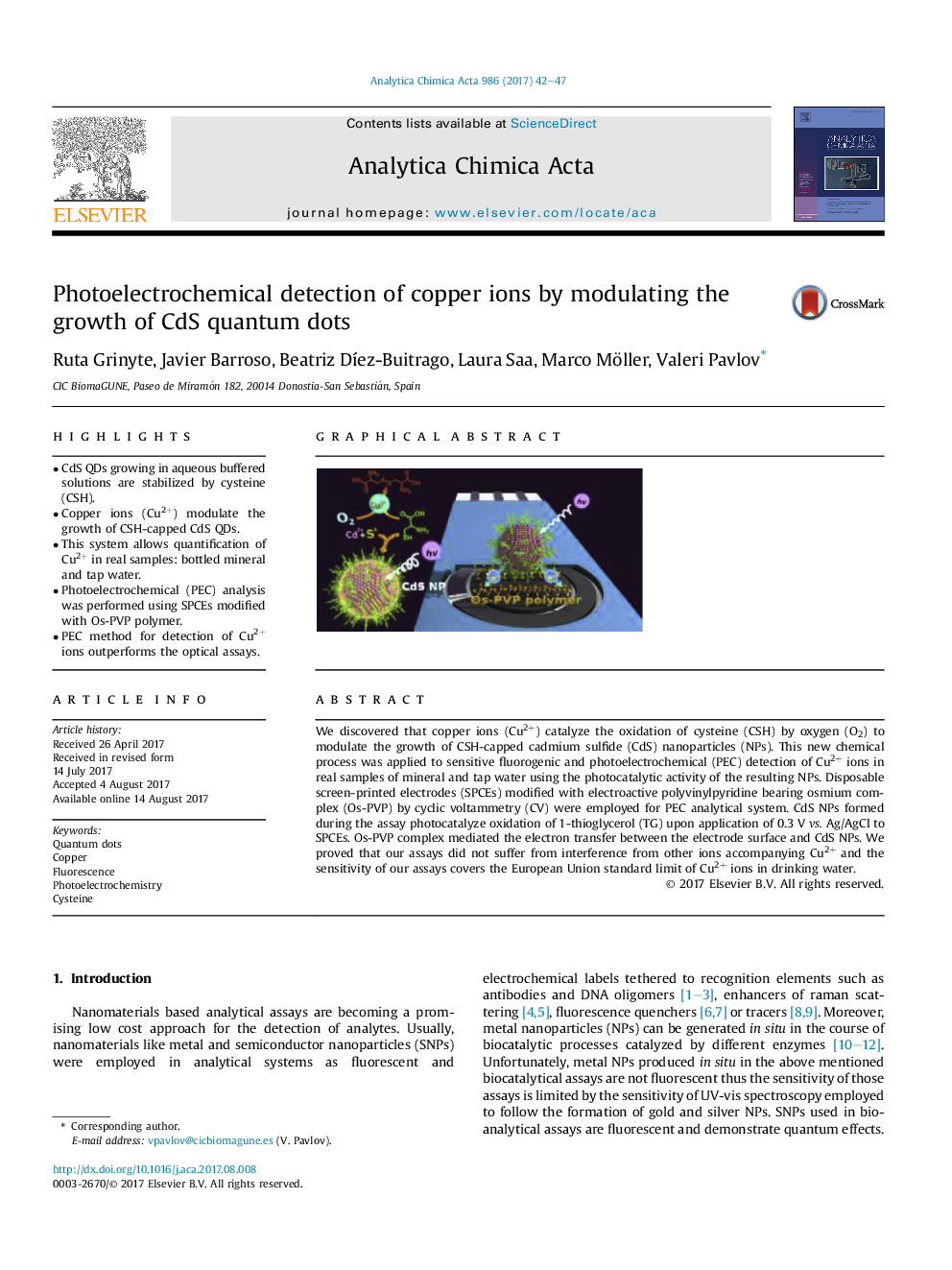
We discovered that copper ions (Cu2+) catalyze the oxidation of cysteine (CSH) by oxygen (O2) to modulate the growth of CSH-capped cadmium sulfide (CdS) nanoparticles (NPs). This new chemical process was applied to sensitive fluorogenic and photoelectrochemical (PEC) detection of Cu2+ ions in real samples of mineral and tap water using the photocatalytic activity of the resulting NPs. Disposable screen-printed electrodes (SPCEs) modified with electroactive polyvinylpyridine bearing osmium complex (Os-PVP) by cyclic voltammetry (CV) were employed for PEC analytical system. CdS NPs formed during the assay photocatalyze oxidation of 1-thioglycerol (TG) upon application of 0.3Â V vs. Ag/AgCl to SPCEs. Os-PVP complex mediated the electron transfer between the electrode surface and CdS NPs. We proved that our assays did not suffer from interference from other ions accompanying Cu2+ and the sensitivity of our assays covers the European Union standard limit of Cu2+ ions in drinking water.
Graphical abstractModulating growth of CdS nanoparticles by copper as redox catalyst allows designing very simple and sensitive assays using disposable photoelectrochemical screen-printed electrodes (SPCEs) sensitized by conductive osmium complex.Download high-res image (274KB)Download full-size image