| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5130672 | Analytica Chimica Acta | 2017 | 7 Pages |
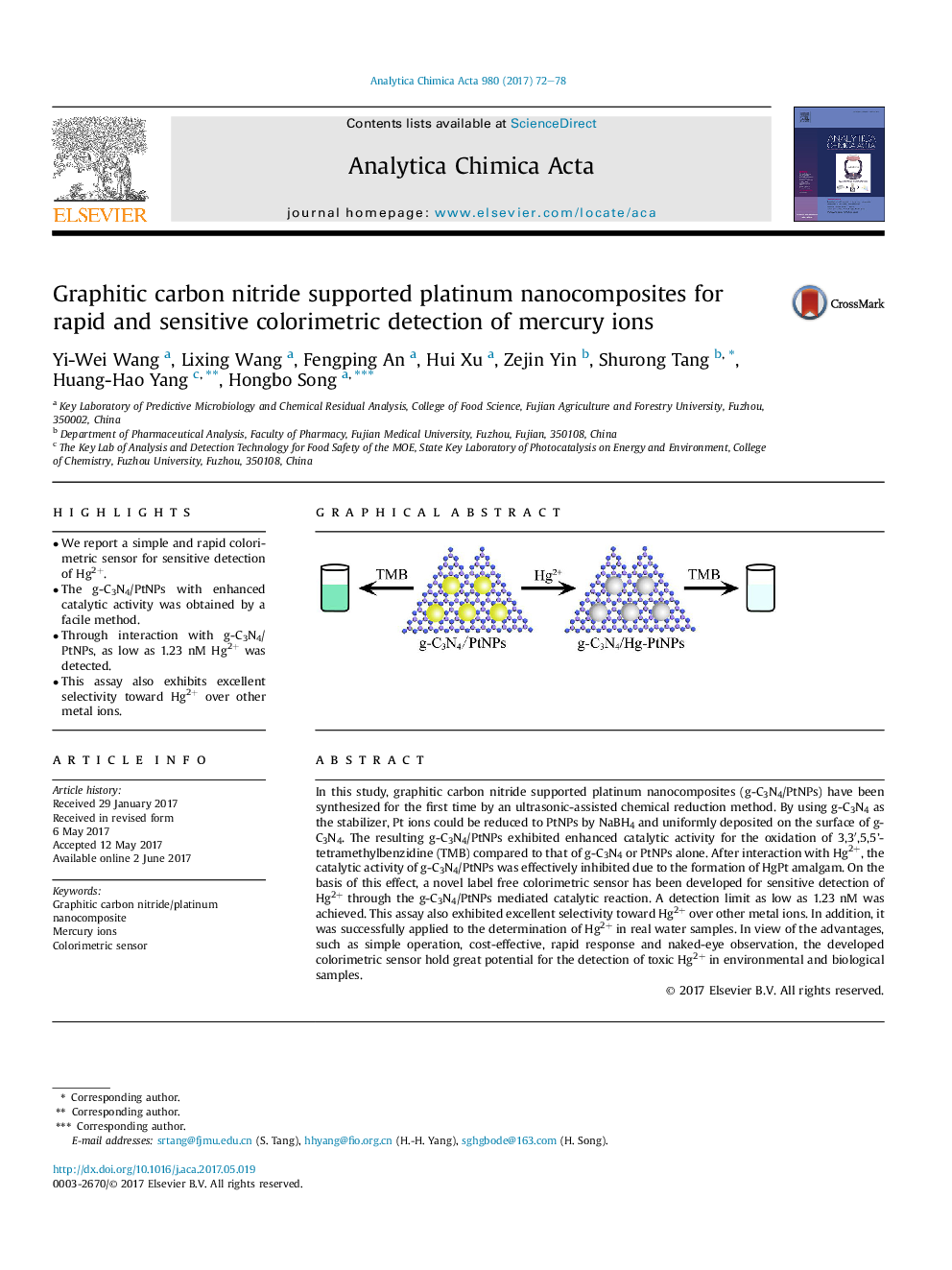
â¢We report a simple and rapid colorimetric sensor for sensitive detection of Hg2+.â¢The g-C3N4/PtNPs with enhanced catalytic activity was obtained by a facile method.â¢Through interaction with g-C3N4/PtNPs, as low as 1.23 nM Hg2+ was detected.â¢This assay also exhibits excellent selectivity toward Hg2+ over other metal ions.
In this study, graphitic carbon nitride supported platinum nanocomposites (g-C3N4/PtNPs) have been synthesized for the first time by an ultrasonic-assisted chemical reduction method. By using g-C3N4 as the stabilizer, Pt ions could be reduced to PtNPs by NaBH4 and uniformly deposited on the surface of g-C3N4. The resulting g-C3N4/PtNPs exhibited enhanced catalytic activity for the oxidation of 3,3â²,5,5'-tetramethylbenzidine (TMB) compared to that of g-C3N4 or PtNPs alone. After interaction with Hg2+, the catalytic activity of g-C3N4/PtNPs was effectively inhibited due to the formation of HgPt amalgam. On the basis of this effect, a novel label free colorimetric sensor has been developed for sensitive detection of Hg2+ through the g-C3N4/PtNPs mediated catalytic reaction. A detection limit as low as 1.23Â nM was achieved. This assay also exhibited excellent selectivity toward Hg2+ over other metal ions. In addition, it was successfully applied to the determination of Hg2+ in real water samples. In view of the advantages, such as simple operation, cost-effective, rapid response and naked-eye observation, the developed colorimetric sensor hold great potential for the detection of toxic Hg2+ in environmental and biological samples.
Graphical abstractDownload high-res image (244KB)Download full-size image