| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5130786 | Analytica Chimica Acta | 2017 | 10 Pages |
â¢A novel sensor for ultrasensitive detection of PA in aqueous media by dual mechanism.â¢The limit of detection of sensor 4 is as low as 0.57 ppb PA in aqueous media.â¢Sensor 4 can detect PA as highly sensitive as 11.46 ag cmâ2 by contact mode.â¢It is practically applicable for sensing PA in real samples and vapor phase.
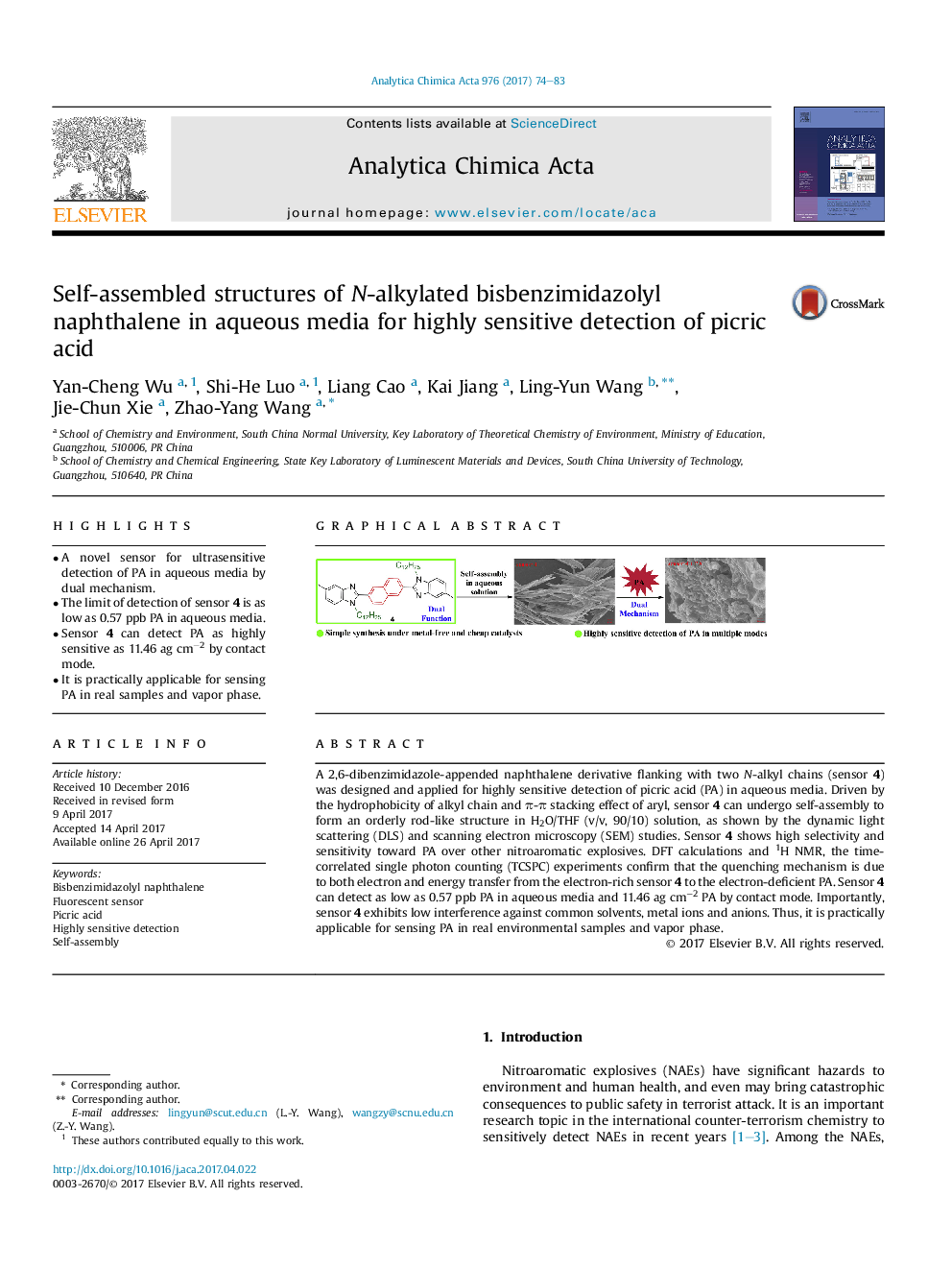
A 2,6-dibenzimidazole-appended naphthalene derivative flanking with two N-alkyl chains (sensor 4) was designed and applied for highly sensitive detection of picric acid (PA) in aqueous media. Driven by the hydrophobicity of alkyl chain and Ï-Ï stacking effect of aryl, sensor 4 can undergo self-assembly to form an orderly rod-like structure in H2O/THF (v/v, 90/10) solution, as shown by the dynamic light scattering (DLS) and scanning electron microscopy (SEM) studies. Sensor 4 shows high selectivity and sensitivity toward PA over other nitroaromatic explosives. DFT calculations and 1H NMR, the time-correlated single photon counting (TCSPC) experiments confirm that the quenching mechanism is due to both electron and energy transfer from the electron-rich sensor 4 to the electron-deficient PA. Sensor 4 can detect as low as 0.57 ppb PA in aqueous media and 11.46 ag cmâ2 PA by contact mode. Importantly, sensor 4 exhibits low interference against common solvents, metal ions and anions. Thus, it is practically applicable for sensing PA in real environmental samples and vapor phase.
Graphical abstractDownload high-res image (238KB)Download full-size image