| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5131139 | Analytica Chimica Acta | 2017 | 8 Pages |
â¢Ovalbumin recognition/electron-transfer peptides were immobilized on magnetic beads.â¢The accumulation of the protein through the peptides on the beads caused the change of electrode response.â¢The magnetic beads could be reused for sensing of ovalbumin.
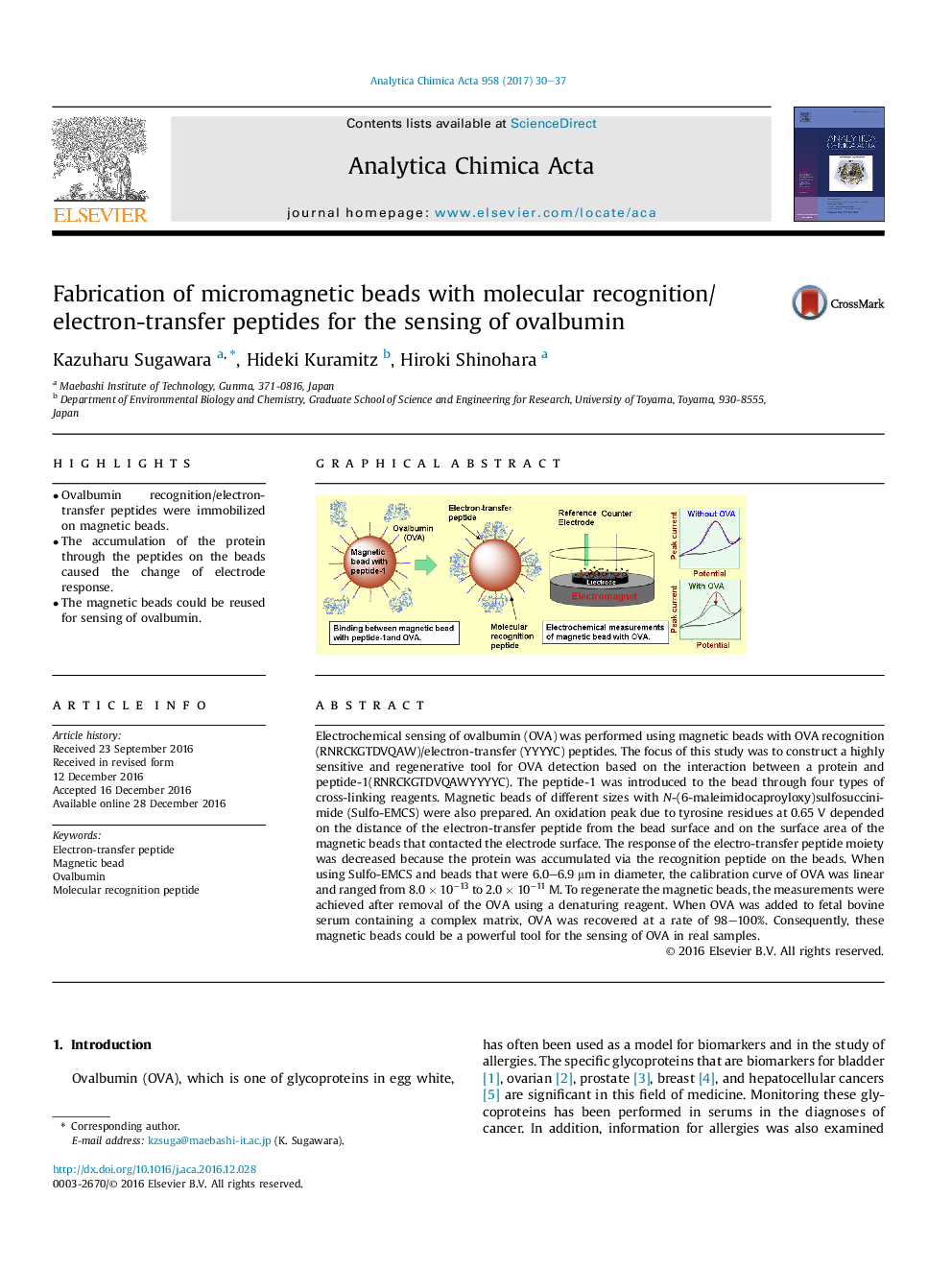
Electrochemical sensing of ovalbumin (OVA) was performed using magnetic beads with OVA recognition (RNRCKGTDVQAW)/electron-transfer (YYYYC) peptides. The focus of this study was to construct a highly sensitive and regenerative tool for OVA detection based on the interaction between a protein and peptide-1(RNRCKGTDVQAWYYYYC). The peptide-1 was introduced to the bead through four types of cross-linking reagents. Magnetic beads of different sizes with N-(6-maleimidocaproyloxy)sulfosuccinimide (Sulfo-EMCS) were also prepared. An oxidation peak due to tyrosine residues at 0.65 V depended on the distance of the electron-transfer peptide from the bead surface and on the surface area of the magnetic beads that contacted the electrode surface. The response of the electro-transfer peptide moiety was decreased because the protein was accumulated via the recognition peptide on the beads. When using Sulfo-EMCS and beads that were 6.0-6.9 μm in diameter, the calibration curve of OVA was linear and ranged from 8.0 Ã 10â13 to 2.0 Ã 10â11 M. To regenerate the magnetic beads, the measurements were achieved after removal of the OVA using a denaturing reagent. When OVA was added to fetal bovine serum containing a complex matrix, OVA was recovered at a rate of 98-100%. Consequently, these magnetic beads could be a powerful tool for the sensing of OVA in real samples.
Graphical abstractDownload high-res image (326KB)Download full-size image