| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5131290 | Analytica Chimica Acta | 2016 | 6 Pages |
â¢We have proposed a simple, high selective and sensitive fluorescent biosensor for ALP.â¢The biosensor combines the high selectivity and the click reaction and the high sensitivity of the fluorescence detection.â¢The biosensor has been successfully applied to detect ALP in serum samples with satisfied results.
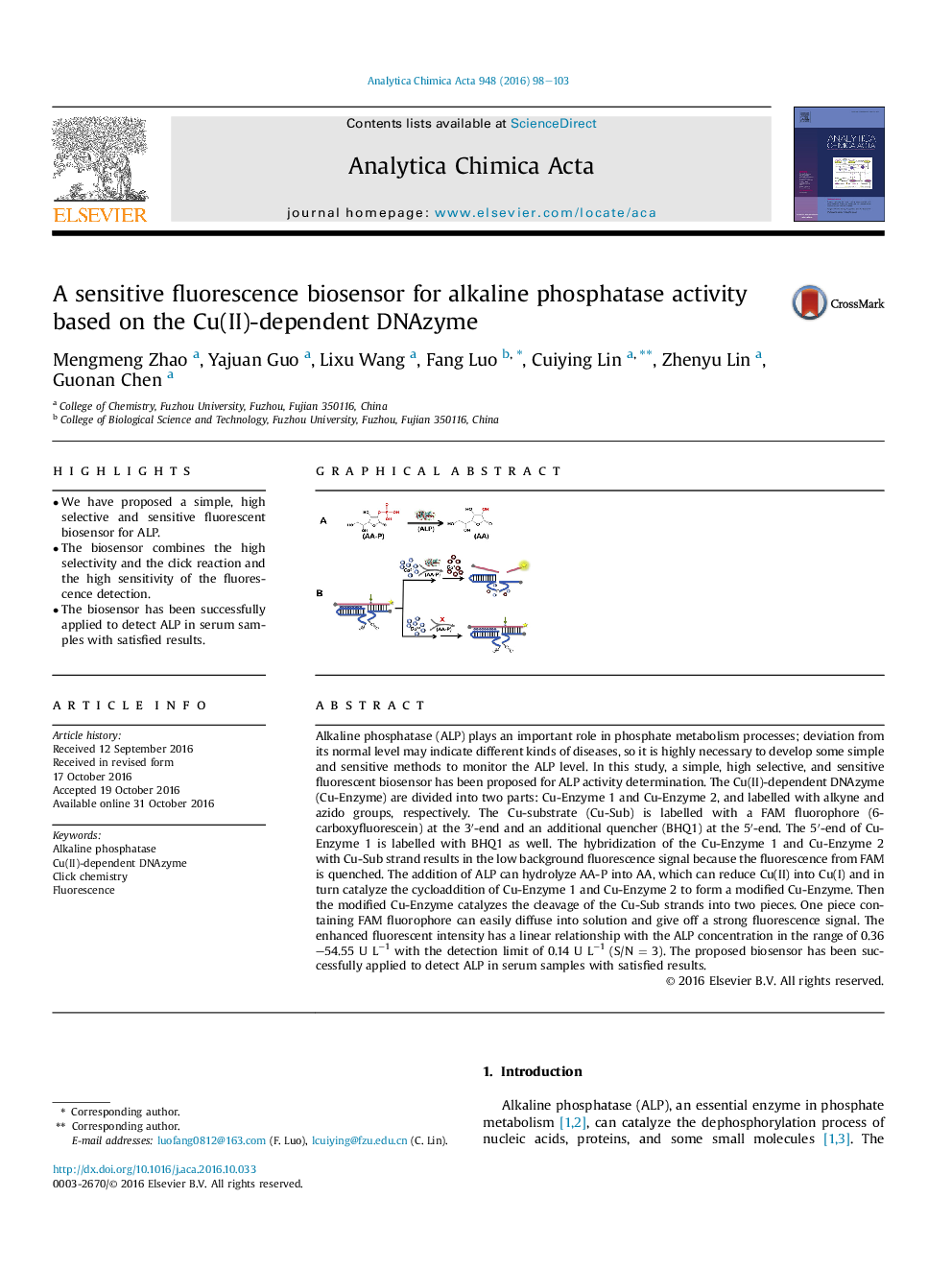
Alkaline phosphatase (ALP) plays an important role in phosphate metabolism processes; deviation from its normal level may indicate different kinds of diseases, so it is highly necessary to develop some simple and sensitive methods to monitor the ALP level. In this study, a simple, high selective, and sensitive fluorescent biosensor has been proposed for ALP activity determination. The Cu(II)-dependent DNAzyme (Cu-Enzyme) are divided into two parts: Cu-Enzyme 1 and Cu-Enzyme 2, and labelled with alkyne and azido groups, respectively. The Cu-substrate (Cu-Sub) is labelled with a FAM fluorophore (6-carboxyfluorescein) at the 3â²-end and an additional quencher (BHQ1) at the 5â²-end. The 5â²-end of Cu-Enzyme 1 is labelled with BHQ1 as well. The hybridization of the Cu-Enzyme 1 and Cu-Enzyme 2 with Cu-Sub strand results in the low background fluorescence signal because the fluorescence from FAM is quenched. The addition of ALP can hydrolyze AA-P into AA, which can reduce Cu(II) into Cu(I) and in turn catalyze the cycloaddition of Cu-Enzyme 1 and Cu-Enzyme 2 to form a modified Cu-Enzyme. Then the modified Cu-Enzyme catalyzes the cleavage of the Cu-Sub strands into two pieces. One piece containing FAM fluorophore can easily diffuse into solution and give off a strong fluorescence signal. The enhanced fluorescent intensity has a linear relationship with the ALP concentration in the range of 0.36-54.55Â UÂ Lâ1 with the detection limit of 0.14Â UÂ Lâ1Â (S/NÂ =Â 3). The proposed biosensor has been successfully applied to detect ALP in serum samples with satisfied results.
Graphical abstractDownload full-size image