| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5131338 | Analytica Chimica Acta | 2016 | 8 Pages |
â¢3-Chloro-4-methyl functionality enhanced enantioselectivities for CD clicked stationary phases.â¢Correlation study of chromatography separation with molecular simulation.â¢Enantioseparation of 39 racemates in multimode HPLLC.
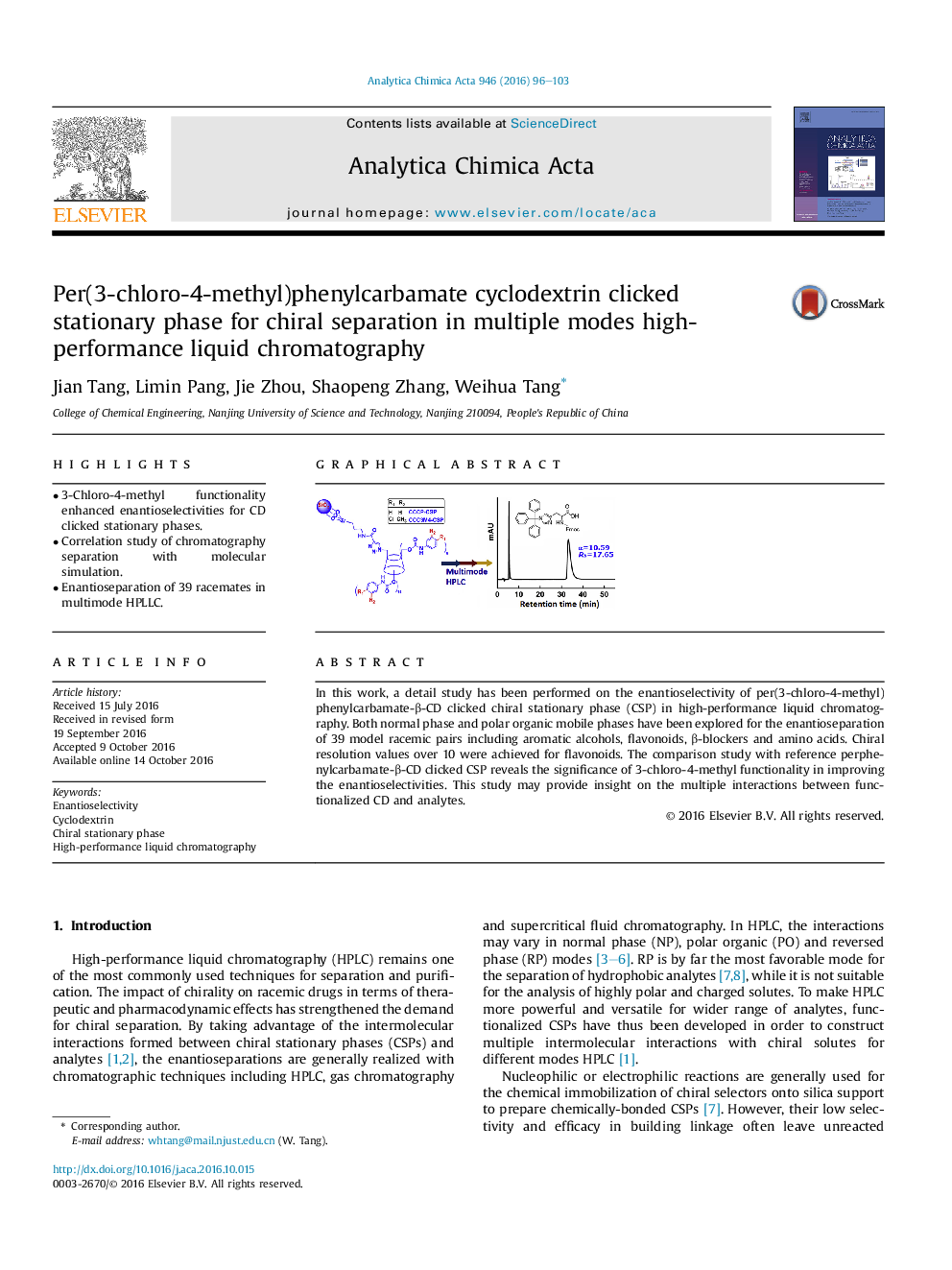
In this work, a detail study has been performed on the enantioselectivity of per(3-chloro-4-methyl)phenylcarbamate-β-CD clicked chiral stationary phase (CSP) in high-performance liquid chromatography. Both normal phase and polar organic mobile phases have been explored for the enantioseparation of 39 model racemic pairs including aromatic alcohols, flavonoids, β-blockers and amino acids. Chiral resolution values over 10 were achieved for flavonoids. The comparison study with reference perphenylcarbamate-β-CD clicked CSP reveals the significance of 3-chloro-4-methyl functionality in improving the enantioselectivities. This study may provide insight on the multiple interactions between functionalized CD and analytes.
Graphical abstractDownload high-res image (204KB)Download full-size image