| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5274489 | Tetrahedron Letters | 2008 | 5 Pages |
Abstract
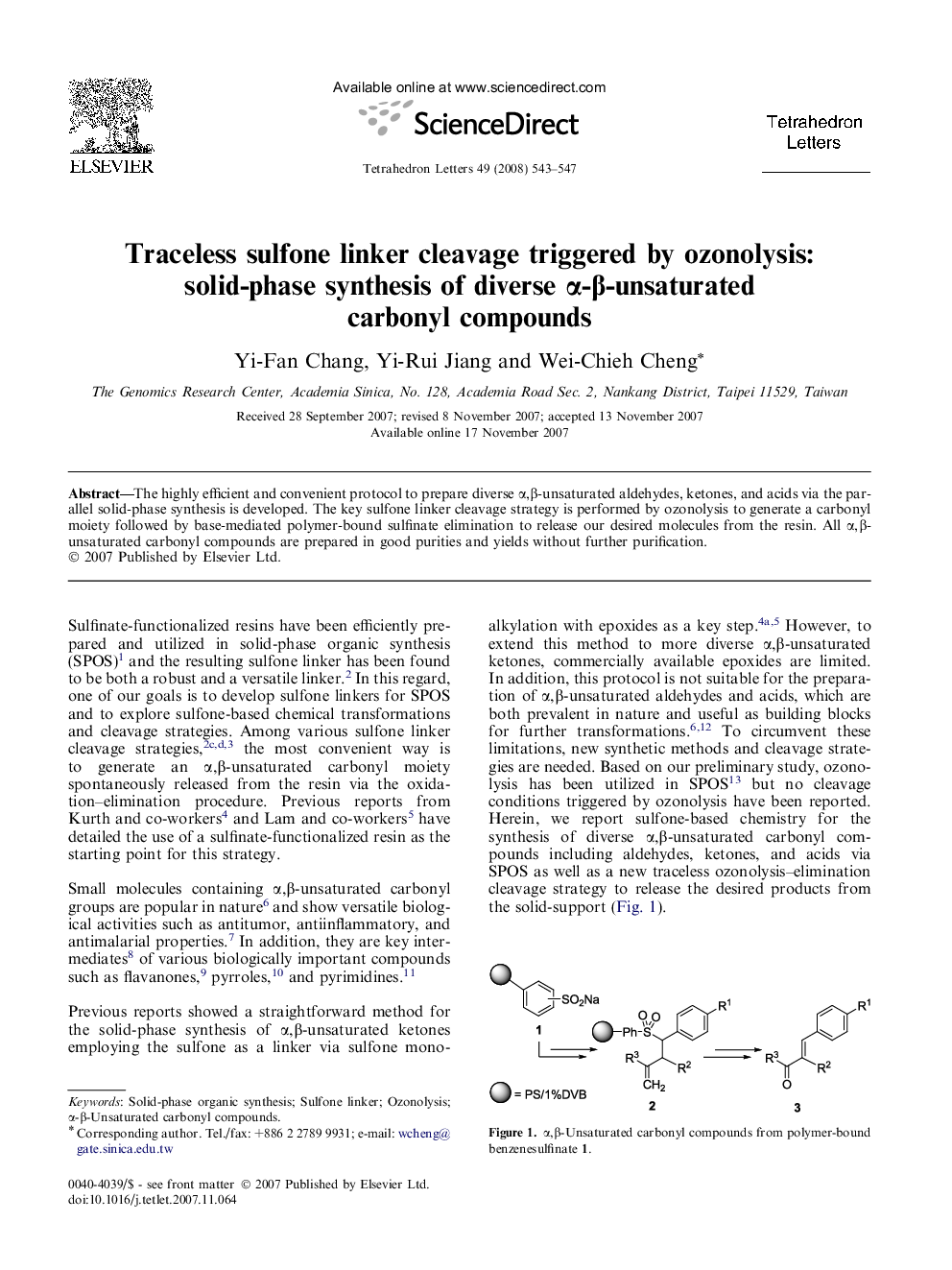
The highly efficient and convenient protocol to prepare diverse α,β-unsaturated aldehydes, ketones, and acids via the parallel solid-phase synthesis is developed. The key sulfone linker cleavage strategy is performed by ozonolysis to generate a carbonyl moiety followed by base-mediated polymer-bound sulfinate elimination to release our desired molecules from the resin. All α,β-unsaturated carbonyl compounds are prepared in good purities and yields without further purification.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Yi-Fan Chang, Yi-Rui Jiang, Wei-Chieh Cheng,