| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5282029 | Tetrahedron Letters | 2006 | 4 Pages |
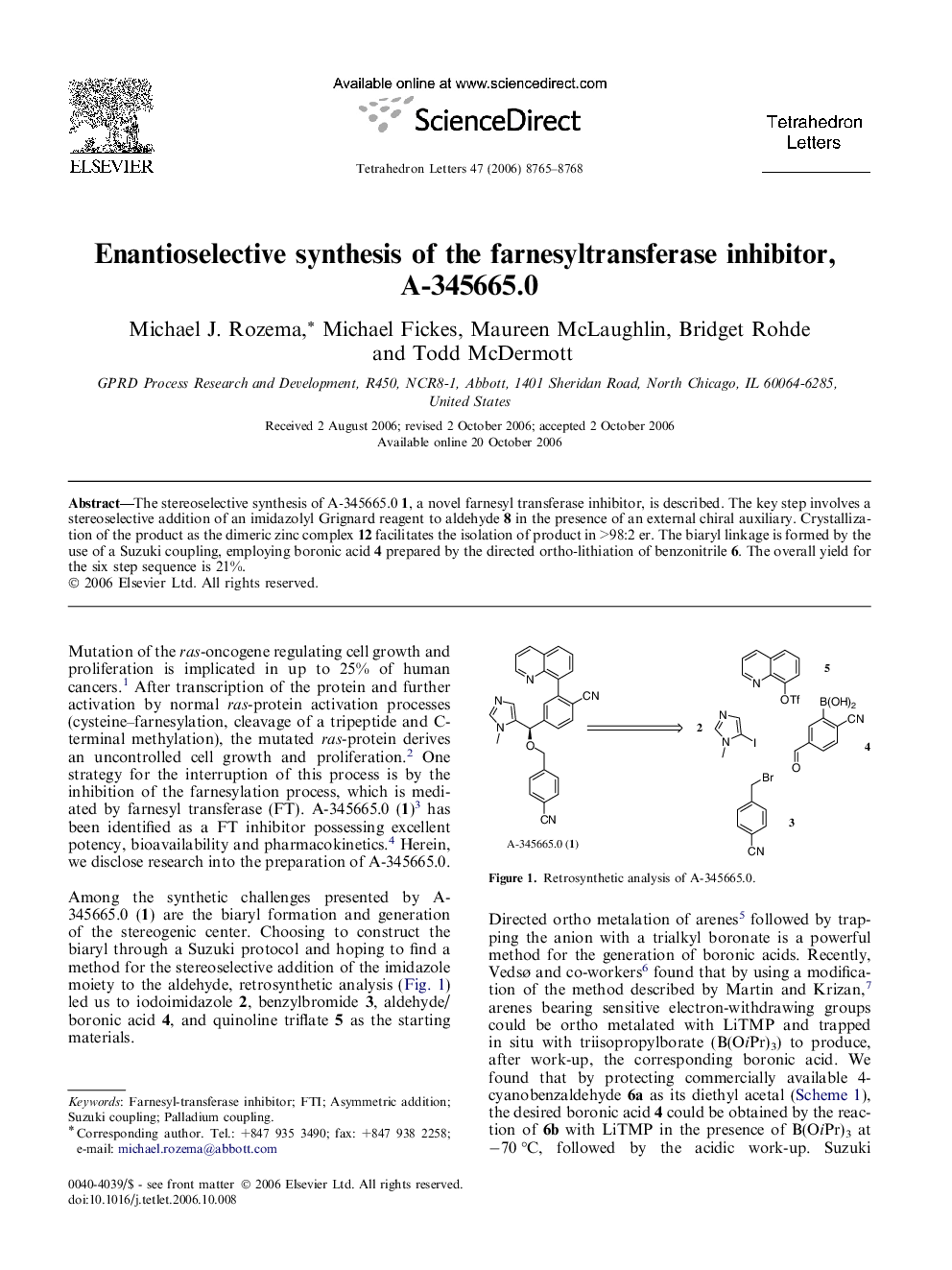
The stereoselective synthesis of A-345665.0 1, a novel farnesyl transferase inhibitor, is described. The key step involves a stereoselective addition of an imidazolyl Grignard reagent to aldehyde 8 in the presence of an external chiral auxiliary. Crystallization of the product as the dimeric zinc complex 12 facilitates the isolation of product in >98:2 er. The biaryl linkage is formed by the use of a Suzuki coupling, employing boronic acid 4 prepared by the directed ortho-lithiation of benzonitrile 6. The overall yield for the six step sequence is 21%.
Graphical abstractThe asymmetric synthesis of A-345665.0, an inhibitor of farnesyl transferase is presented. It is highlighted by the enantioselective addition of an imidazolyl Grignard reagent to an aldehyde in the presence of an external chiral auxiliary and an efficient Suzuki reaction of a boronic acid prepared through the DOM of a benzonitrile.Download full-size image