| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 591638 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2016 | 7 Pages |
•Highly monodisperse hollow Fe3O4@C nanoparticles were prepared with controllable particle sizes.•Hollow Fe3O4@C colloidal suspension has a wider tunable range of the optical spectrum and higher diffraction intensity.•Hollow Fe3O4@C nanoparticles have a number of advantages as a design material for responsive photonic crystal, like low density, better optical properties and high optical contrast.
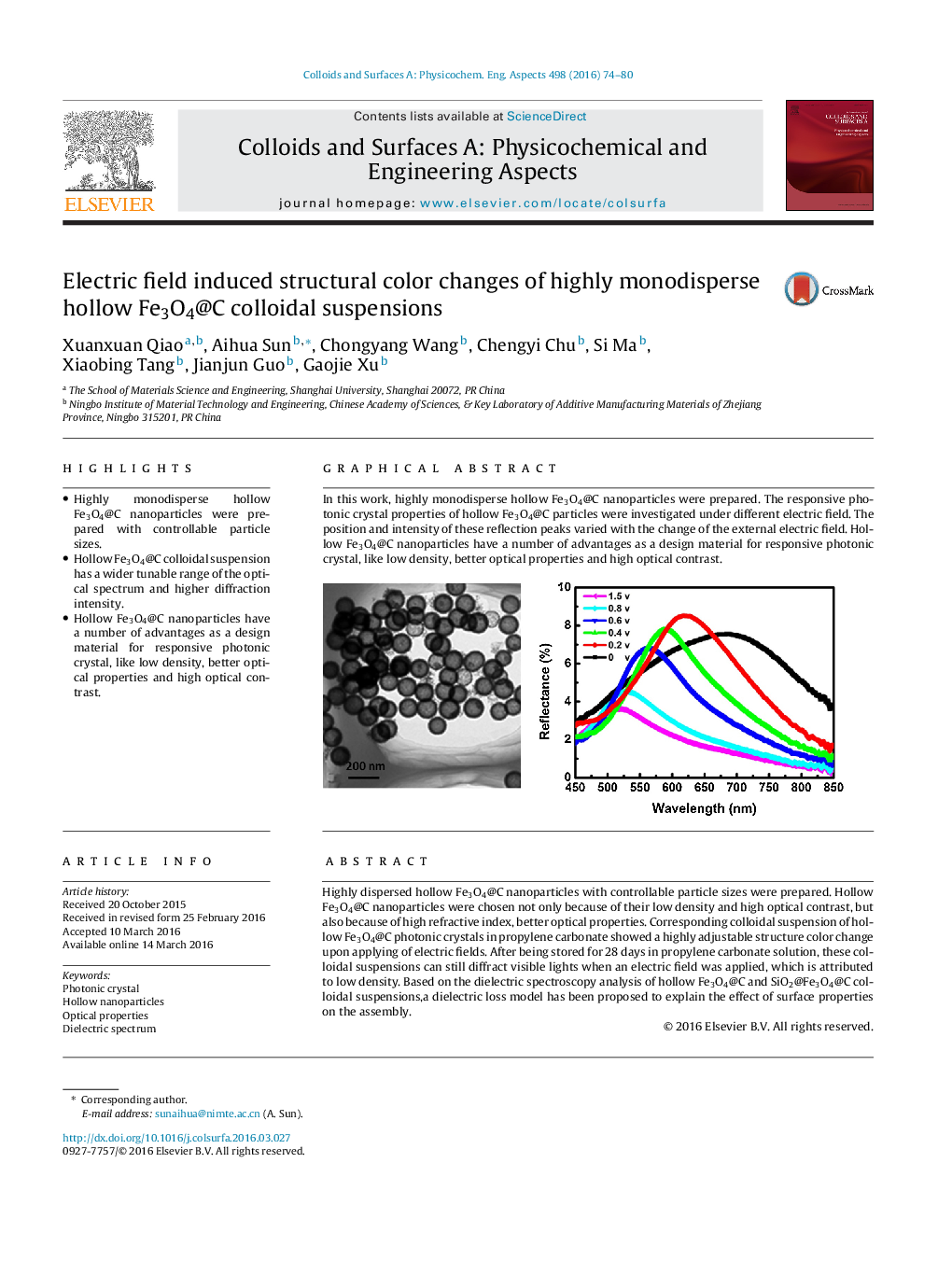
Highly dispersed hollow Fe3O4@C nanoparticles with controllable particle sizes were prepared. Hollow Fe3O4@C nanoparticles were chosen not only because of their low density and high optical contrast, but also because of high refractive index, better optical properties. Corresponding colloidal suspension of hollow Fe3O4@C photonic crystals in propylene carbonate showed a highly adjustable structure color change upon applying of electric fields. After being stored for 28 days in propylene carbonate solution, these colloidal suspensions can still diffract visible lights when an electric field was applied, which is attributed to low density. Based on the dielectric spectroscopy analysis of hollow Fe3O4@C and SiO2@Fe3O4@C colloidal suspensions,a dielectric loss model has been proposed to explain the effect of surface properties on the assembly.
Graphical abstractIn this work, highly monodisperse hollow Fe3O4@C nanoparticles were prepared. The responsive photonic crystal properties of hollow Fe3O4@C particles were investigated under different electric field. The position and intensity of these reflection peaks varied with the change of the external electric field. Hollow Fe3O4@C nanoparticles have a number of advantages as a design material for responsive photonic crystal, like low density, better optical properties and high optical contrast.Figure optionsDownload full-size imageDownload as PowerPoint slide