| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 591730 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2016 | 9 Pages |
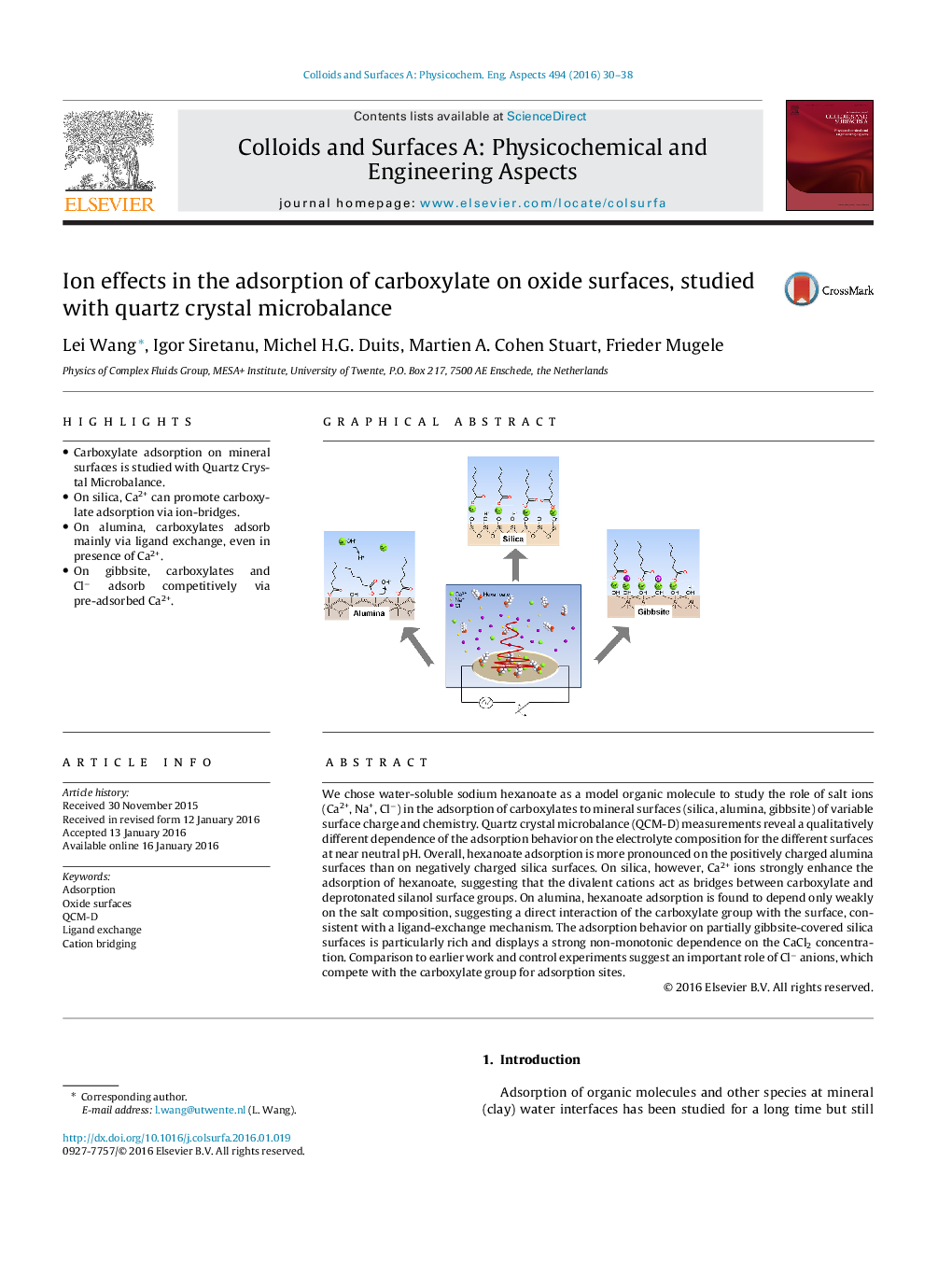
•Carboxylate adsorption on mineral surfaces is studied with Quartz Crystal Microbalance.•On silica, Ca2+ can promote carboxylate adsorption via ion-bridges.•On alumina, carboxylates adsorb mainly via ligand exchange, even in presence of Ca2+.•On gibbsite, carboxylates and Cl− adsorb competitively via pre-adsorbed Ca2+.
We chose water-soluble sodium hexanoate as a model organic molecule to study the role of salt ions (Ca2+, Na+, Cl−) in the adsorption of carboxylates to mineral surfaces (silica, alumina, gibbsite) of variable surface charge and chemistry. Quartz crystal microbalance (QCM-D) measurements reveal a qualitatively different dependence of the adsorption behavior on the electrolyte composition for the different surfaces at near neutral pH. Overall, hexanoate adsorption is more pronounced on the positively charged alumina surfaces than on negatively charged silica surfaces. On silica, however, Ca2+ ions strongly enhance the adsorption of hexanoate, suggesting that the divalent cations act as bridges between carboxylate and deprotonated silanol surface groups. On alumina, hexanoate adsorption is found to depend only weakly on the salt composition, suggesting a direct interaction of the carboxylate group with the surface, consistent with a ligand-exchange mechanism. The adsorption behavior on partially gibbsite-covered silica surfaces is particularly rich and displays a strong non-monotonic dependence on the CaCl2 concentration. Comparison to earlier work and control experiments suggest an important role of Cl− anions, which compete with the carboxylate group for adsorption sites.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide