| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 591732 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2016 | 10 Pages |
•Phase behaviour for catalytic reactions in microemulsion systems is investigated.•In hydroformulation reactions, phase boundaries are shifted during reaction.•In Heck reactions, one-phase systems are possible at low reactant concentrations.•High inorganic base concentrations lead to two-phase systems.
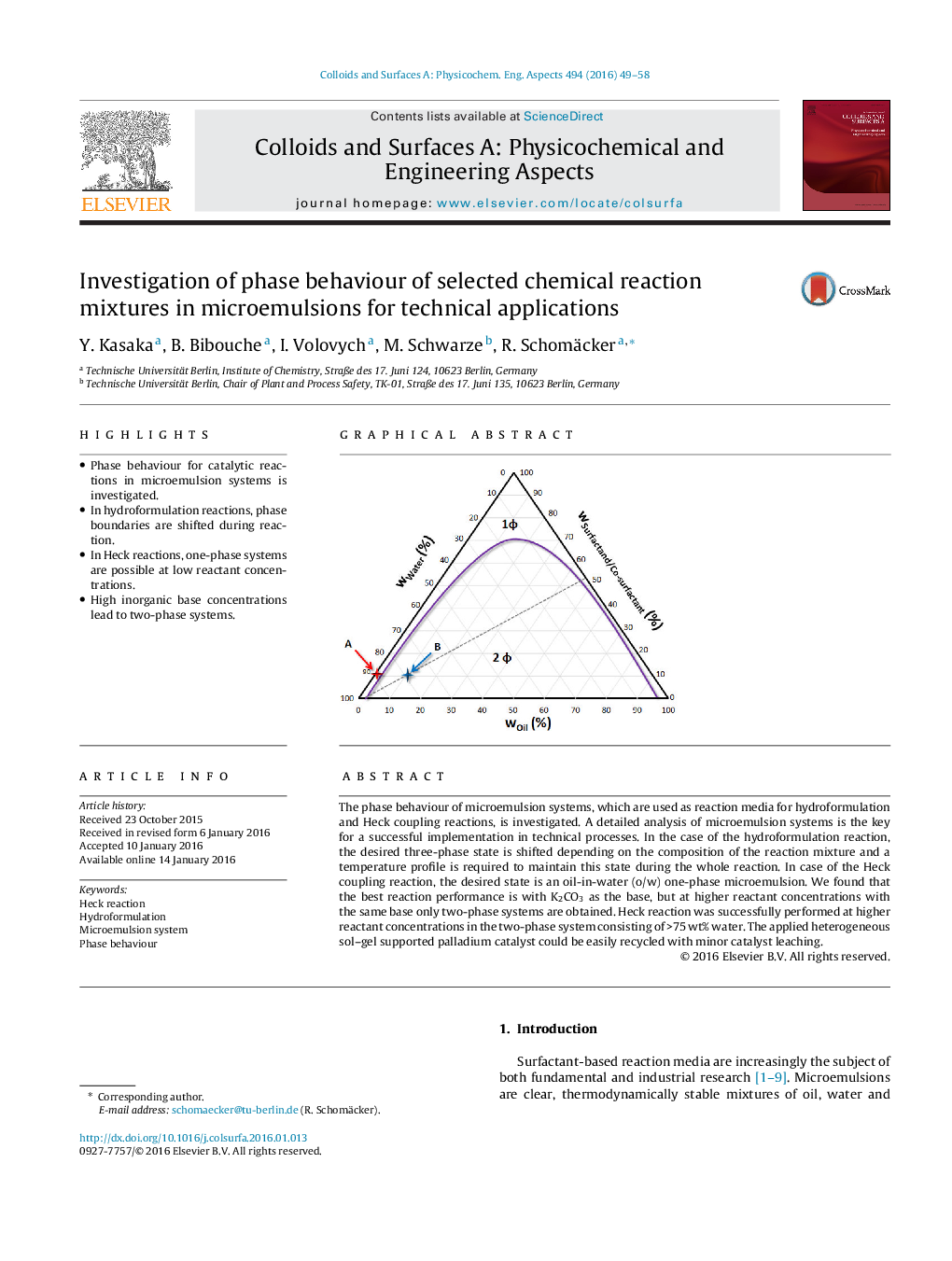
The phase behaviour of microemulsion systems, which are used as reaction media for hydroformulation and Heck coupling reactions, is investigated. A detailed analysis of microemulsion systems is the key for a successful implementation in technical processes. In the case of the hydroformulation reaction, the desired three-phase state is shifted depending on the composition of the reaction mixture and a temperature profile is required to maintain this state during the whole reaction. In case of the Heck coupling reaction, the desired state is an oil-in-water (o/w) one-phase microemulsion. We found that the best reaction performance is with K2CO3 as the base, but at higher reactant concentrations with the same base only two-phase systems are obtained. Heck reaction was successfully performed at higher reactant concentrations in the two-phase system consisting of >75 wt% water. The applied heterogeneous sol–gel supported palladium catalyst could be easily recycled with minor catalyst leaching.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide