| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 591750 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2016 | 7 Pages |
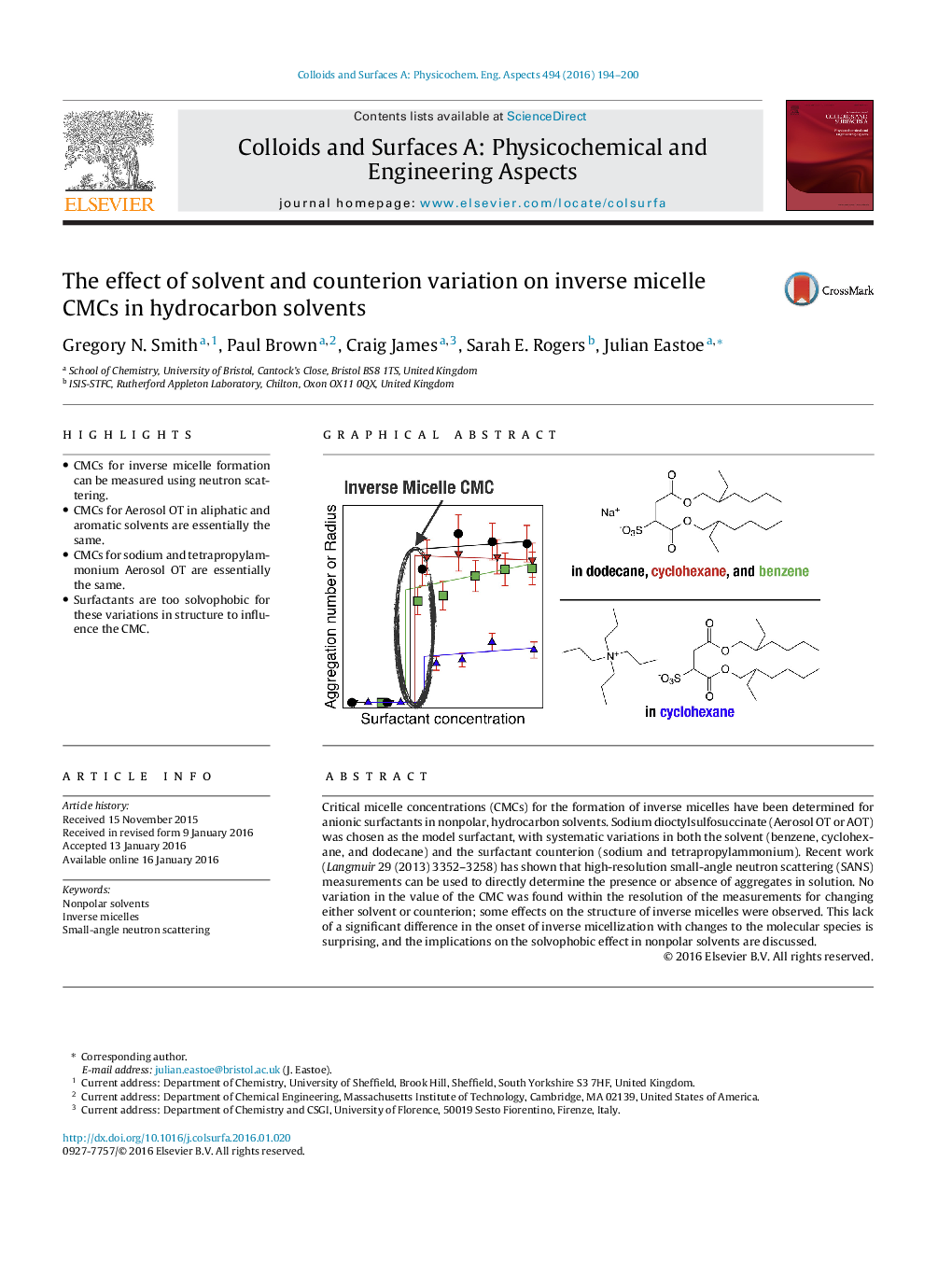
•CMCs for inverse micelle formation can be measured using neutron scattering.•CMCs for Aerosol OT in aliphatic and aromatic solvents are essentially the same.•CMCs for sodium and tetrapropylammonium Aerosol OT are essentially the same.•Surfactants are too solvophobic for these variations in structure to influence the CMC.
Critical micelle concentrations (CMCs) for the formation of inverse micelles have been determined for anionic surfactants in nonpolar, hydrocarbon solvents. Sodium dioctylsulfosuccinate (Aerosol OT or AOT) was chosen as the model surfactant, with systematic variations in both the solvent (benzene, cyclohexane, and dodecane) and the surfactant counterion (sodium and tetrapropylammonium). Recent work (Langmuir 29 (2013) 3352–3258) has shown that high-resolution small-angle neutron scattering (SANS) measurements can be used to directly determine the presence or absence of aggregates in solution. No variation in the value of the CMC was found within the resolution of the measurements for changing either solvent or counterion; some effects on the structure of inverse micelles were observed. This lack of a significant difference in the onset of inverse micellization with changes to the molecular species is surprising, and the implications on the solvophobic effect in nonpolar solvents are discussed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide