| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 591765 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2016 | 8 Pages |
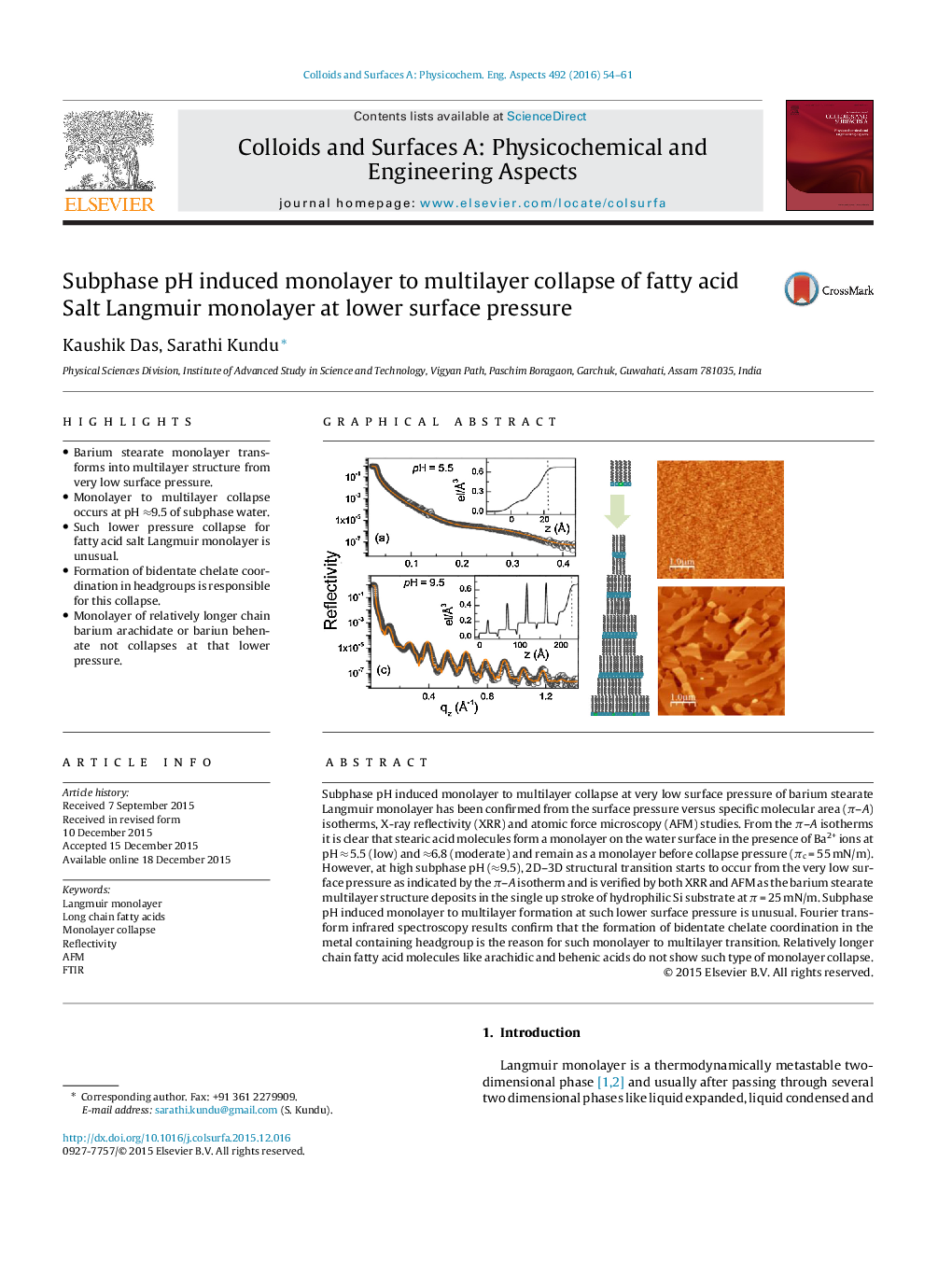
•Barium stearate monolayer transforms into multilayer structure from very low surface pressure.•Monolayer to multilayer collapse occurs at pH ≈9.5 of subphase water.•Such lower pressure collapse for fatty acid salt Langmuir monolayer is unusual.•Formation of bidentate chelate coordination in headgroups is responsible for this collapse.•Monolayer of relatively longer chain barium arachidate or bariun behenate not collapses at that lower pressure.
Subphase pH induced monolayer to multilayer collapse at very low surface pressure of barium stearate Langmuir monolayer has been confirmed from the surface pressure versus specific molecular area (π–A) isotherms, X-ray reflectivity (XRR) and atomic force microscopy (AFM) studies. From the π–A isotherms it is clear that stearic acid molecules form a monolayer on the water surface in the presence of Ba2+ ions at pH ≈ 5.5 (low) and ≈6.8 (moderate) and remain as a monolayer before collapse pressure (πc = 55 mN/m). However, at high subphase pH (≈9.5), 2D–3D structural transition starts to occur from the very low surface pressure as indicated by the π–A isotherm and is verified by both XRR and AFM as the barium stearate multilayer structure deposits in the single up stroke of hydrophilic Si substrate at π = 25 mN/m. Subphase pH induced monolayer to multilayer formation at such lower surface pressure is unusual. Fourier transform infrared spectroscopy results confirm that the formation of bidentate chelate coordination in the metal containing headgroup is the reason for such monolayer to multilayer transition. Relatively longer chain fatty acid molecules like arachidic and behenic acids do not show such type of monolayer collapse.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide