| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 591804 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2016 | 11 Pages |
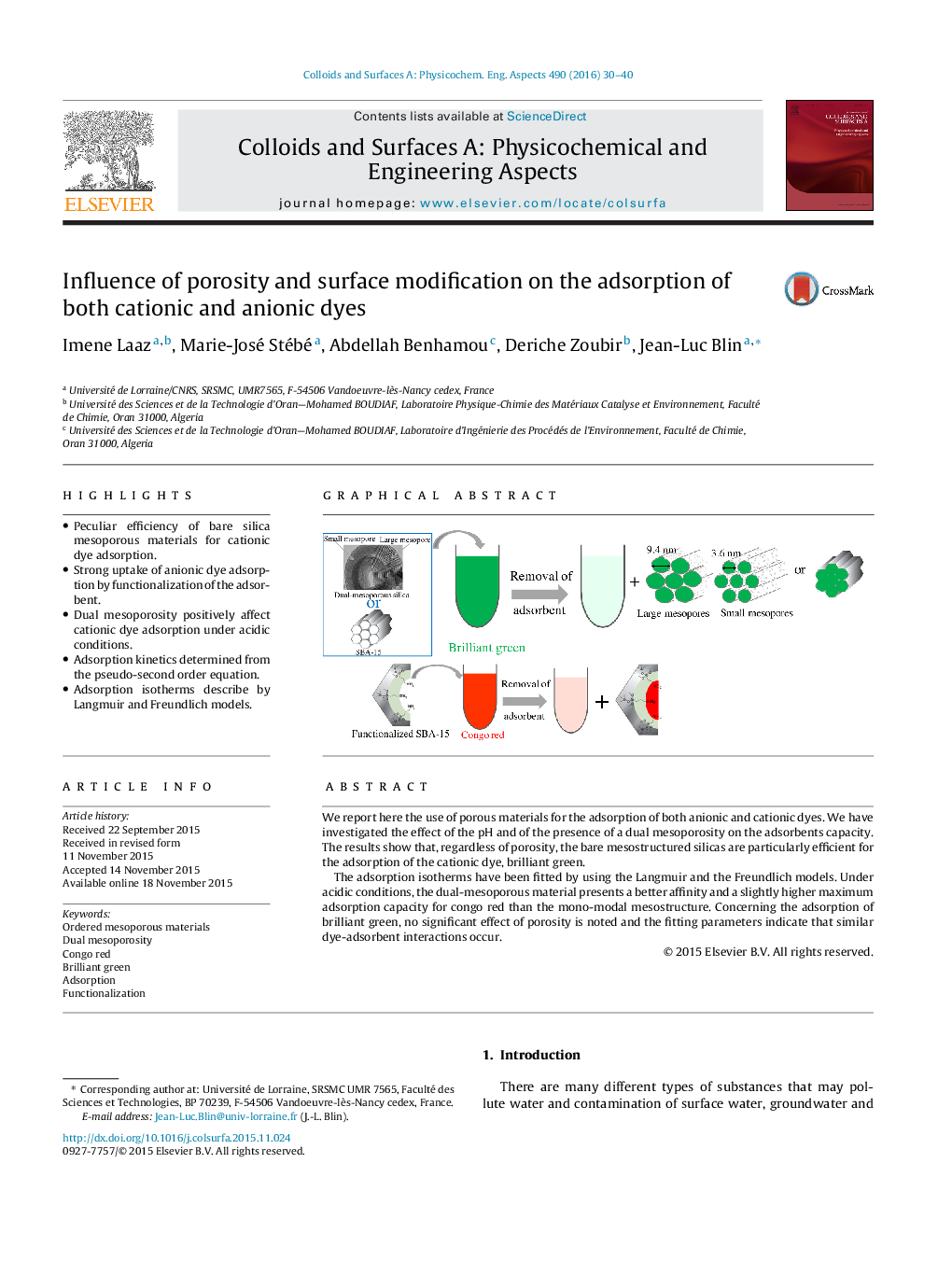
•Peculiar efficiency of bare silica mesoporous materials for cationic dye adsorption.•Strong uptake of anionic dye adsorption by functionalization of the adsorbent.•Dual mesoporosity positively affect cationic dye adsorption under acidic conditions.•Adsorption kinetics determined from the pseudo-second order equation.•Adsorption isotherms describe by Langmuir and Freundlich models.
We report here the use of porous materials for the adsorption of both anionic and cationic dyes. We have investigated the effect of the pH and of the presence of a dual mesoporosity on the adsorbents capacity. The results show that, regardless of porosity, the bare mesostructured silicas are particularly efficient for the adsorption of the cationic dye, brilliant green.The adsorption isotherms have been fitted by using the Langmuir and the Freundlich models. Under acidic conditions, the dual-mesoporous material presents a better affinity and a slightly higher maximum adsorption capacity for congo red than the mono-modal mesostructure. Concerning the adsorption of brilliant green, no significant effect of porosity is noted and the fitting parameters indicate that similar dye-adsorbent interactions occur.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide