| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 591925 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2015 | 9 Pages |
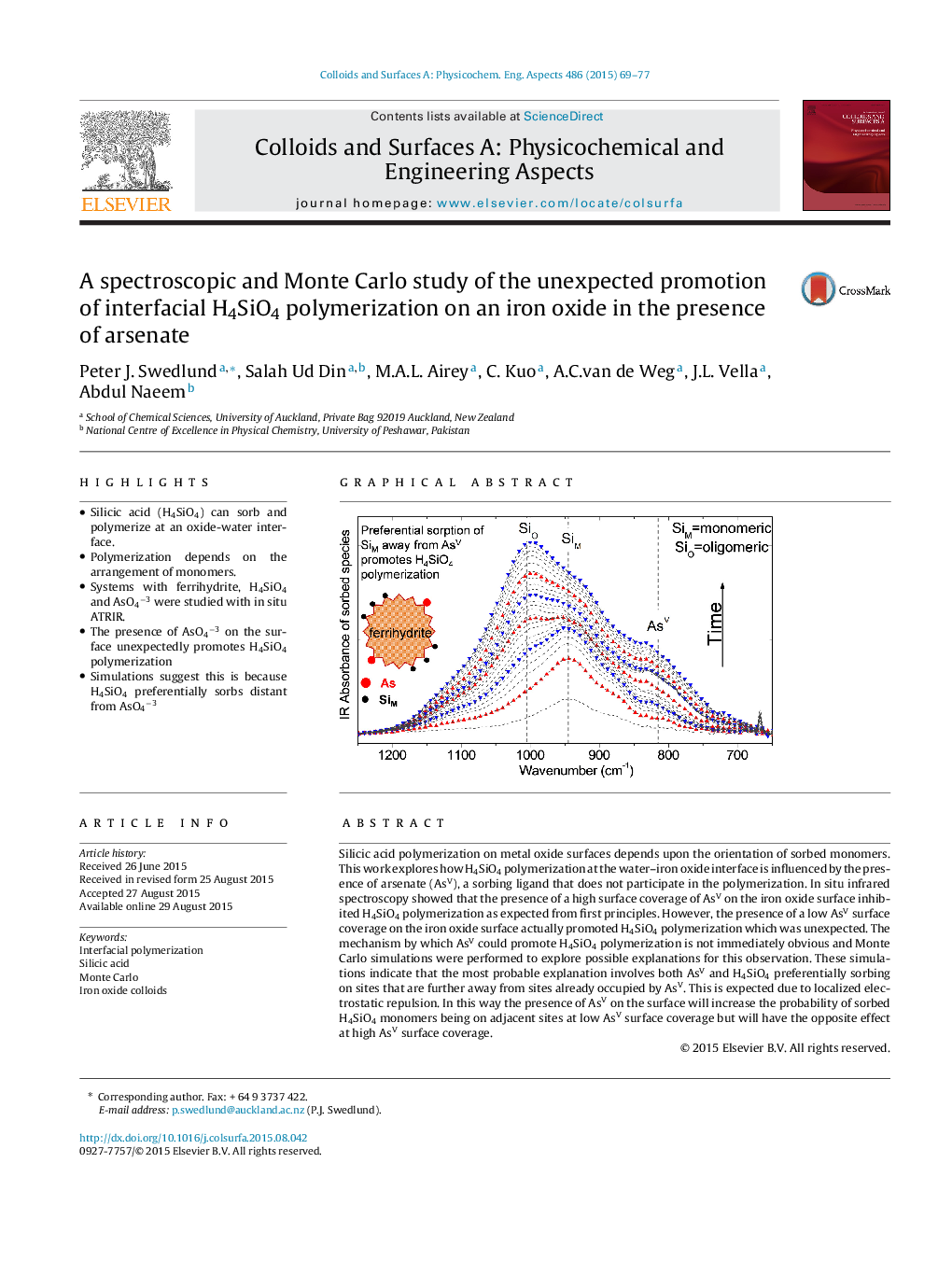
•Silicic acid (H4SiO4) can sorb and polymerize at an oxide-water interface.•Polymerization depends on the arrangement of monomers.•Systems with ferrihydrite, H4SiO4 and AsO4−3 were studied with in situ ATRIR.•The presence of AsO4−3 on the surface unexpectedly promotes H4SiO4 polymerization•Simulations suggest this is because H4SiO4 preferentially sorbs distant from AsO4−3
Silicic acid polymerization on metal oxide surfaces depends upon the orientation of sorbed monomers. This work explores how H4SiO4 polymerization at the water–iron oxide interface is influenced by the presence of arsenate (AsV), a sorbing ligand that does not participate in the polymerization. In situ infrared spectroscopy showed that the presence of a high surface coverage of AsV on the iron oxide surface inhibited H4SiO4 polymerization as expected from first principles. However, the presence of a low AsV surface coverage on the iron oxide surface actually promoted H4SiO4 polymerization which was unexpected. The mechanism by which AsV could promote H4SiO4 polymerization is not immediately obvious and Monte Carlo simulations were performed to explore possible explanations for this observation. These simulations indicate that the most probable explanation involves both AsV and H4SiO4 preferentially sorbing on sites that are further away from sites already occupied by AsV. This is expected due to localized electrostatic repulsion. In this way the presence of AsV on the surface will increase the probability of sorbed H4SiO4 monomers being on adjacent sites at low AsV surface coverage but will have the opposite effect at high AsV surface coverage.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide