| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592069 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2015 | 7 Pages |
•A non-covalent bonding method is utilized for the formation of pH-switchable wormlike micelles.•The microstructure transition was the fundamental reason for the pH-switchable rheological properties.•The unusual rheological and micellar responses are caused by the changing binding capacity of a hydrotrope to C16MPBr as the pH varies.
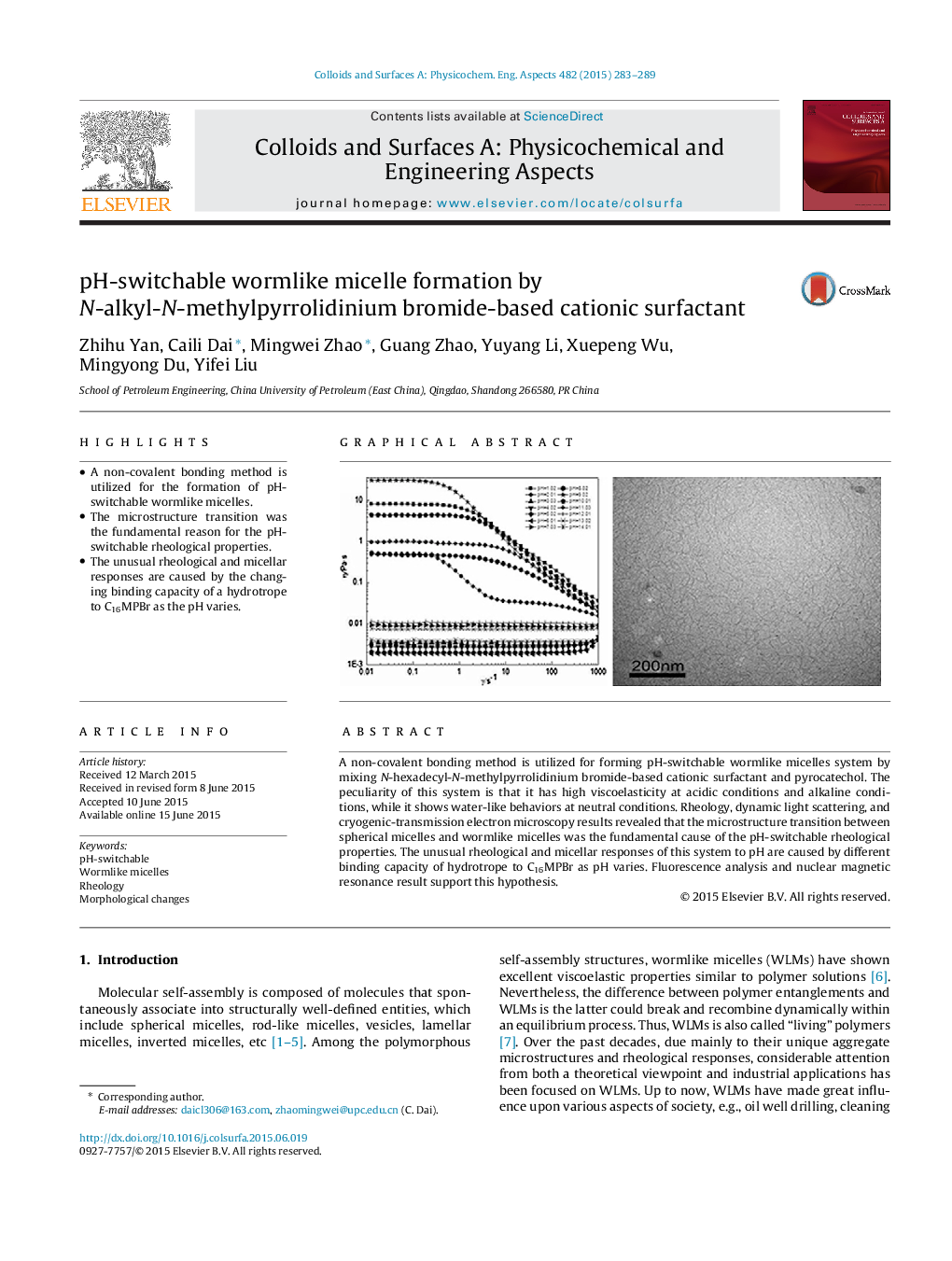
A non-covalent bonding method is utilized for forming pH-switchable wormlike micelles system by mixing N-hexadecyl-N-methylpyrrolidinium bromide-based cationic surfactant and pyrocatechol. The peculiarity of this system is that it has high viscoelasticity at acidic conditions and alkaline conditions, while it shows water-like behaviors at neutral conditions. Rheology, dynamic light scattering, and cryogenic-transmission electron microscopy results revealed that the microstructure transition between spherical micelles and wormlike micelles was the fundamental cause of the pH-switchable rheological properties. The unusual rheological and micellar responses of this system to pH are caused by different binding capacity of hydrotrope to C16MPBr as pH varies. Fluorescence analysis and nuclear magnetic resonance result support this hypothesis.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide