| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592072 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2015 | 9 Pages |
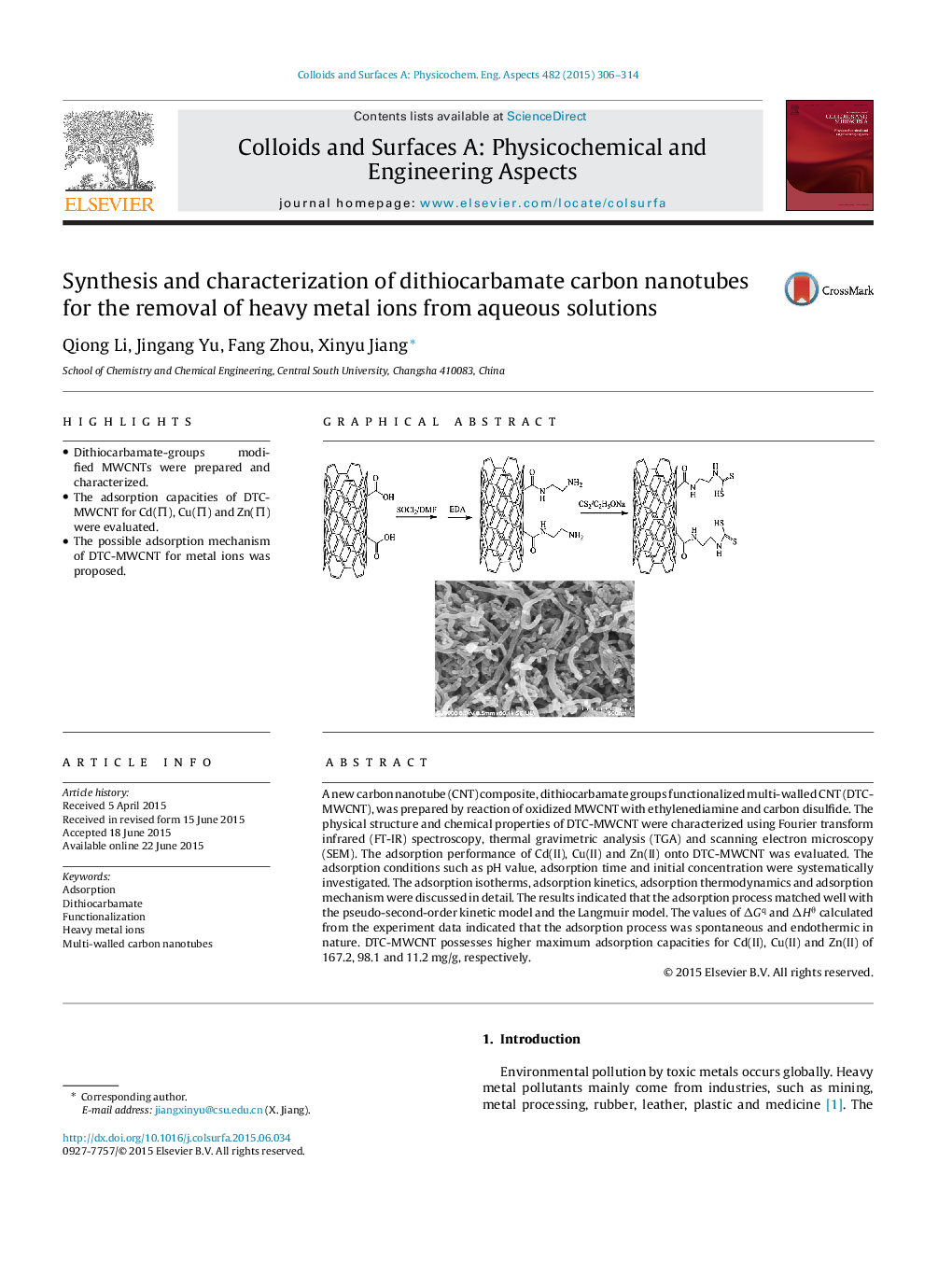
•Dithiocarbamate-groups modified MWCNTs were prepared and characterized.•The adsorption capacities of DTC-MWCNT for Cd(Π), Cu(Π) and Zn(Π) were evaluated.•The possible adsorption mechanism of DTC-MWCNT for metal ions was proposed.
A new carbon nanotube (CNT) composite, dithiocarbamate groups functionalized multi-walled CNT (DTC-MWCNT), was prepared by reaction of oxidized MWCNT with ethylenediamine and carbon disulfide. The physical structure and chemical properties of DTC-MWCNT were characterized using Fourier transform infrared (FT-IR) spectroscopy, thermal gravimetric analysis (TGA) and scanning electron microscopy (SEM). The adsorption performance of Cd(II), Cu(II) and Zn(II) onto DTC-MWCNT was evaluated. The adsorption conditions such as pH value, adsorption time and initial concentration were systematically investigated. The adsorption isotherms, adsorption kinetics, adsorption thermodynamics and adsorption mechanism were discussed in detail. The results indicated that the adsorption process matched well with the pseudo-second-order kinetic model and the Langmuir model. The values of ΔGq and ΔHθ calculated from the experiment data indicated that the adsorption process was spontaneous and endothermic in nature. DTC-MWCNT possesses higher maximum adsorption capacities for Cd(II), Cu(II) and Zn(II) of 167.2, 98.1 and 11.2 mg/g, respectively.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide