| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592090 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2015 | 10 Pages |
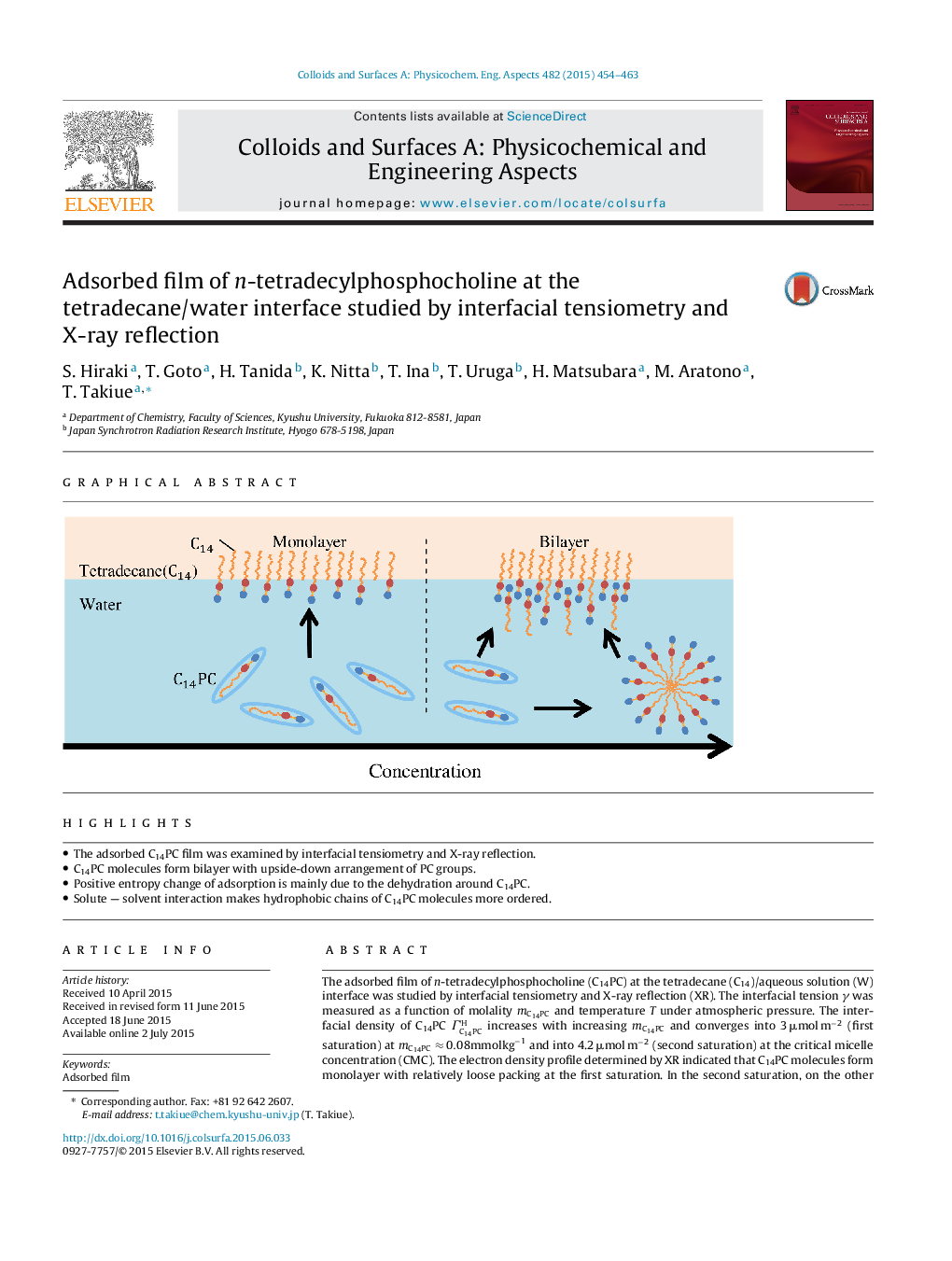
•The adsorbed C14PC film was examined by interfacial tensiometry and X-ray reflection.•C14PC molecules form bilayer with upside-down arrangement of PC groups.•Positive entropy change of adsorption is mainly due to the dehydration around C14PC.•Solute — solvent interaction makes hydrophobic chains of C14PC molecules more ordered.
The adsorbed film of n-tetradecylphosphocholine (C14PC) at the tetradecane (C14)/aqueous solution (W) interface was studied by interfacial tensiometry and X-ray reflection (XR). The interfacial tension γ was measured as a function of molality mC14PCmC14PC and temperature T under atmospheric pressure. The interfacial density of C14PC ΓC14PCH increases with increasing mC14PCmC14PC and converges into 3 μmol m−2 (first saturation) at mC14PC≈0.08mmolkg−1mC14PC≈0.08mmolkg−1 and into 4.2 μmol m−2 (second saturation) at the critical micelle concentration (CMC). The electron density profile determined by XR indicated that C14PC molecules form monolayer with relatively loose packing at the first saturation. In the second saturation, on the other hand, the molecules form bilayer in which charge separated phosphocholine groups take upside-down arrangement to interact attractively between neighbors. The positive entropy changes associated with adsorption both from monomeric and from micellar states result from that the contribution of dehydration around C14PC molecules, especially around PC groups, by the adsorption from aqueous solution overcomes that of a comparatively ordered molecular orientation at the interface. By comparing the results at the C14/W interface with those at the Air (A)/W one, it was shown that in the monolayer and bilayer states, hydrophobic chains of C14PC molecules are more ordered at the C14/W than at the A/W interface because of attractive interaction between C14PC and C14 molecules. This leads to smaller value of the partial molar entropy and energy changes associated with the adsorption at the C14/W than at the A/W interface. Furthermore the entropy of micelle formation changes from positive to negative with increasing T, suggesting that the entropy gain due to the dehydration exceeds the entropy loss by the aggregation at low temperatures, while the gain is less than the loss at high temperature.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide