| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592140 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2015 | 14 Pages |
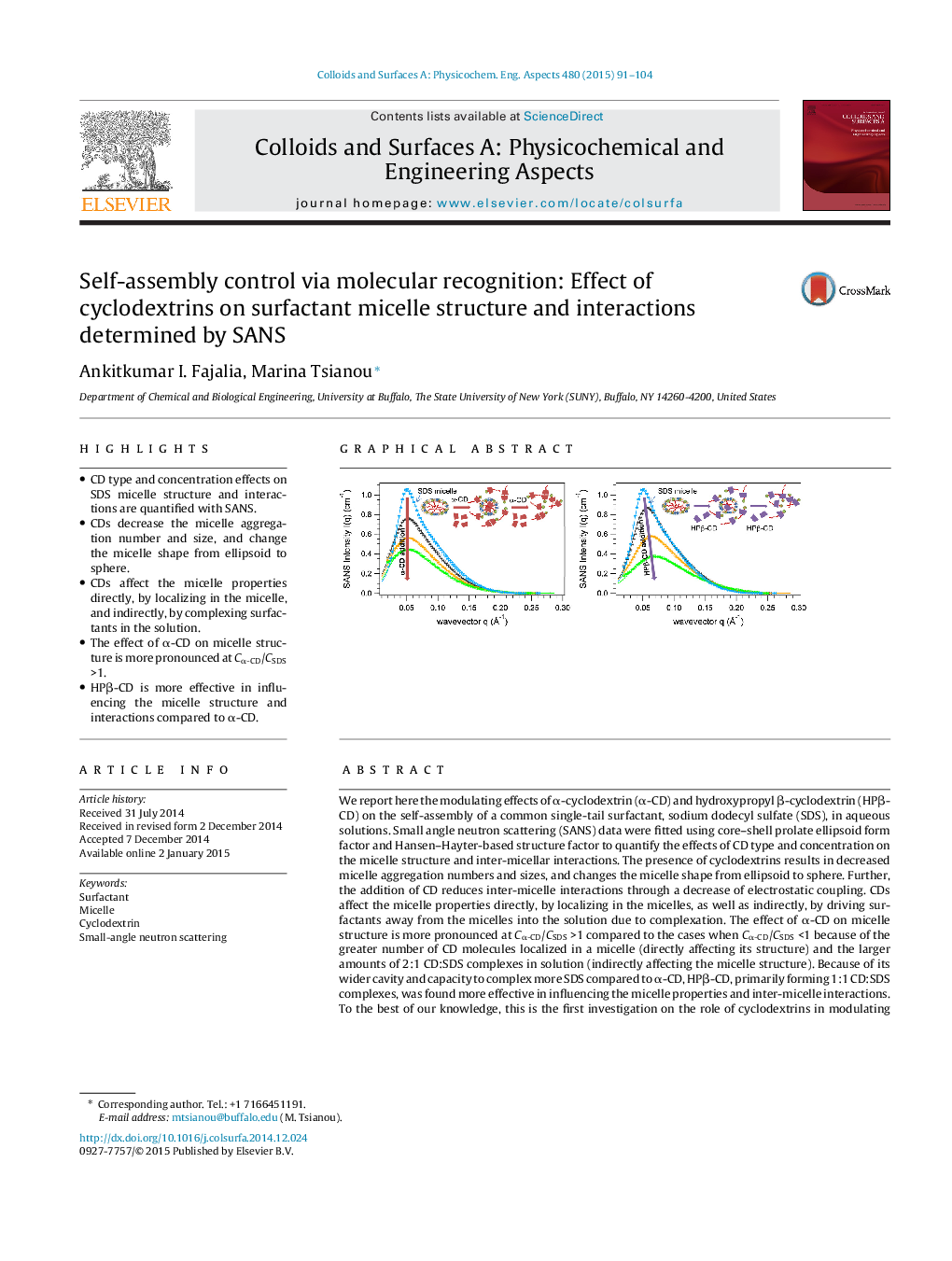
•CD type and concentration effects on SDS micelle structure and interactions are quantified with SANS.•CDs decrease the micelle aggregation number and size, and change the micelle shape from ellipsoid to sphere.•CDs affect the micelle properties directly, by localizing in the micelle, and indirectly, by complexing surfactants in the solution.•The effect of α-CD on micelle structure is more pronounced at Cα-CD/CSDS >1.•HPβ-CD is more effective in influencing the micelle structure and interactions compared to α-CD.
We report here the modulating effects of α-cyclodextrin (α-CD) and hydroxypropyl β-cyclodextrin (HPβ-CD) on the self-assembly of a common single-tail surfactant, sodium dodecyl sulfate (SDS), in aqueous solutions. Small angle neutron scattering (SANS) data were fitted using core–shell prolate ellipsoid form factor and Hansen–Hayter-based structure factor to quantify the effects of CD type and concentration on the micelle structure and inter-micellar interactions. The presence of cyclodextrins results in decreased micelle aggregation numbers and sizes, and changes the micelle shape from ellipsoid to sphere. Further, the addition of CD reduces inter-micelle interactions through a decrease of electrostatic coupling. CDs affect the micelle properties directly, by localizing in the micelles, as well as indirectly, by driving surfactants away from the micelles into the solution due to complexation. The effect of α-CD on micelle structure is more pronounced at Cα-CD/CSDS >1 compared to the cases when Cα-CD/CSDS <1 because of the greater number of CD molecules localized in a micelle (directly affecting its structure) and the larger amounts of 2:1 CD:SDS complexes in solution (indirectly affecting the micelle structure). Because of its wider cavity and capacity to complex more SDS compared to α-CD, HPβ-CD, primarily forming 1:1 CD:SDS complexes, was found more effective in influencing the micelle properties and inter-micelle interactions. To the best of our knowledge, this is the first investigation on the role of cyclodextrins in modulating intra- and inter-micelle structure and interactions. These results support the utilization of modulated surfactant self-assembly for applications such as drug delivery and nanoparticle synthesis.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide