| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592148 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2015 | 6 Pages |
•All-atom molecular dynamics of the SDS aqueous solution is performed.•Diffusivities of aggregates as function of their aggregation numbers are studied.•Comparisons with theoretical and experimental data are made.
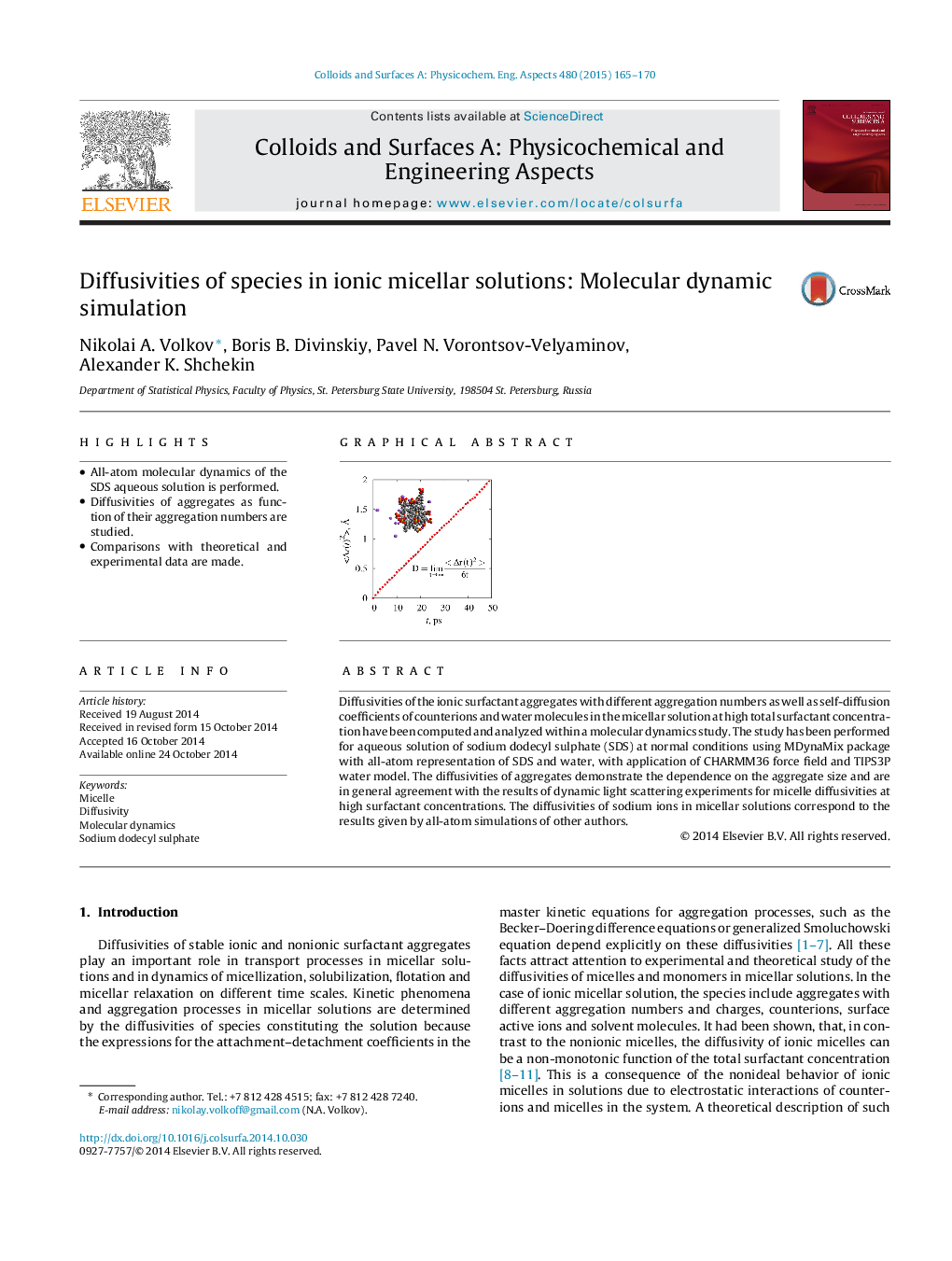
Diffusivities of the ionic surfactant aggregates with different aggregation numbers as well as self-diffusion coefficients of counterions and water molecules in the micellar solution at high total surfactant concentration have been computed and analyzed within a molecular dynamics study. The study has been performed for aqueous solution of sodium dodecyl sulphate (SDS) at normal conditions using MDynaMix package with all-atom representation of SDS and water, with application of CHARMM36 force field and TIPS3P water model. The diffusivities of aggregates demonstrate the dependence on the aggregate size and are in general agreement with the results of dynamic light scattering experiments for micelle diffusivities at high surfactant concentrations. The diffusivities of sodium ions in micellar solutions correspond to the results given by all-atom simulations of other authors.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide