| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592204 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2015 | 10 Pages |
•We study the mechanism of the membrane formation via NIPS combined with gelation.•γ-Butyrolactone (γ-BL) improves the thermodynamic stability of the dope solution.•Liquid–liquid phase separation occurs before gelation at a low PVDF concentration.•Changing DMF/γ-BL ratio can tailor the membrane avoiding the finger-like structure.
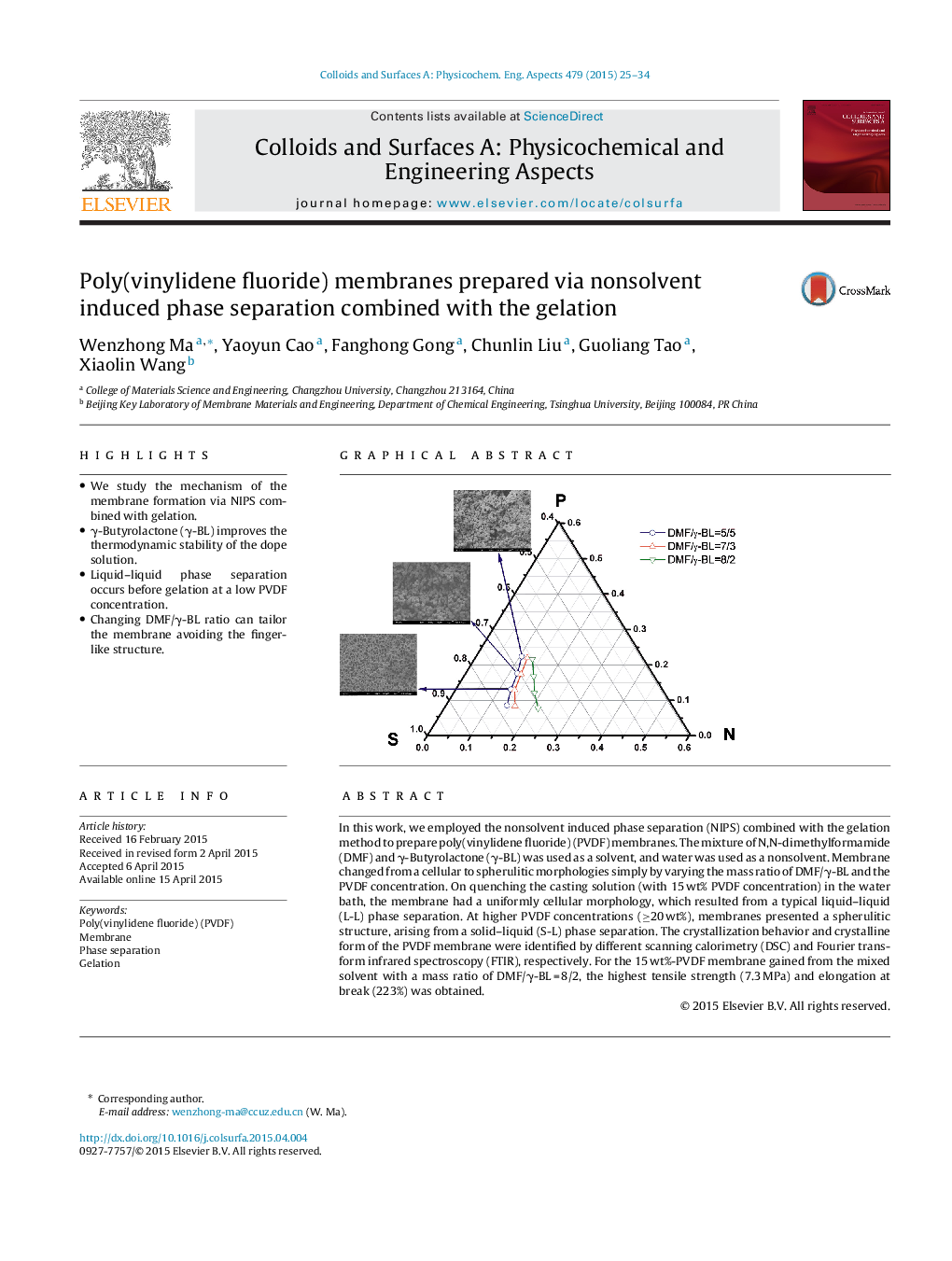
In this work, we employed the nonsolvent induced phase separation (NIPS) combined with the gelation method to prepare poly(vinylidene fluoride) (PVDF) membranes. The mixture of N,N-dimethylformamide (DMF) and γ-Butyrolactone (γ-BL) was used as a solvent, and water was used as a nonsolvent. Membrane changed from a cellular to spherulitic morphologies simply by varying the mass ratio of DMF/γ-BL and the PVDF concentration. On quenching the casting solution (with 15 wt% PVDF concentration) in the water bath, the membrane had a uniformly cellular morphology, which resulted from a typical liquid–liquid (L-L) phase separation. At higher PVDF concentrations (≥20 wt%), membranes presented a spherulitic structure, arising from a solid–liquid (S-L) phase separation. The crystallization behavior and crystalline form of the PVDF membrane were identified by different scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR), respectively. For the 15 wt%-PVDF membrane gained from the mixed solvent with a mass ratio of DMF/γ-BL = 8/2, the highest tensile strength (7.3 MPa) and elongation at break (223%) was obtained.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide