| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592263 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2015 | 7 Pages |
•A novel and cheap approach for producing mesoporous silica is proposed.•The mesoporous silicas have very high surface area which can reach >600 m2 g−1.•The pore structure of mesoporous silicas can be tuned easily.•Bagasse ash, a solid waste of sugar mills, is a viable material to produce mesoporous silicas.
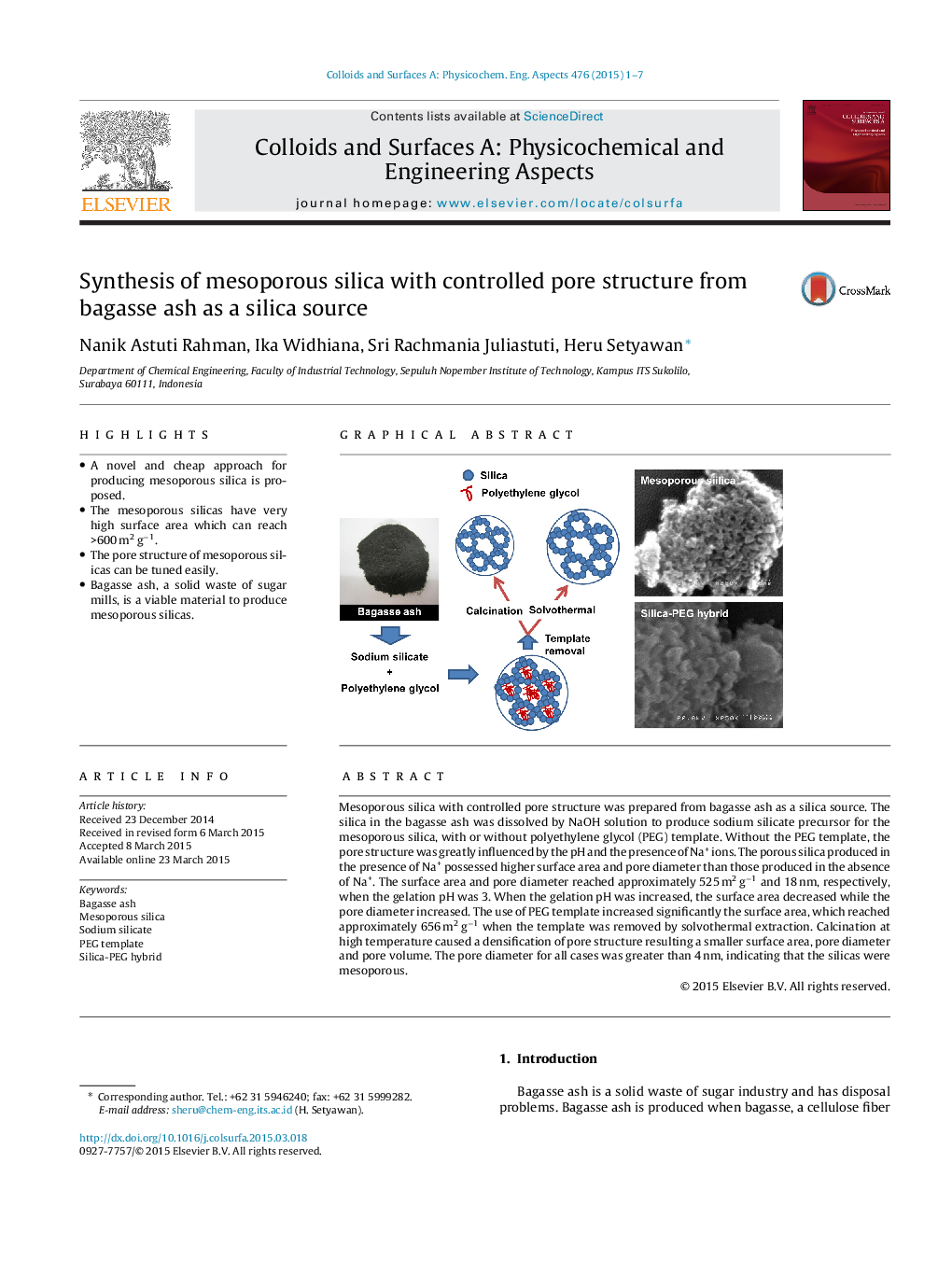
Mesoporous silica with controlled pore structure was prepared from bagasse ash as a silica source. The silica in the bagasse ash was dissolved by NaOH solution to produce sodium silicate precursor for the mesoporous silica, with or without polyethylene glycol (PEG) template. Without the PEG template, the pore structure was greatly influenced by the pH and the presence of Na+ ions. The porous silica produced in the presence of Na+ possessed higher surface area and pore diameter than those produced in the absence of Na+. The surface area and pore diameter reached approximately 525 m2 g−1 and 18 nm, respectively, when the gelation pH was 3. When the gelation pH was increased, the surface area decreased while the pore diameter increased. The use of PEG template increased significantly the surface area, which reached approximately 656 m2 g−1 when the template was removed by solvothermal extraction. Calcination at high temperature caused a densification of pore structure resulting a smaller surface area, pore diameter and pore volume. The pore diameter for all cases was greater than 4 nm, indicating that the silicas were mesoporous.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide