| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592363 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2015 | 5 Pages |
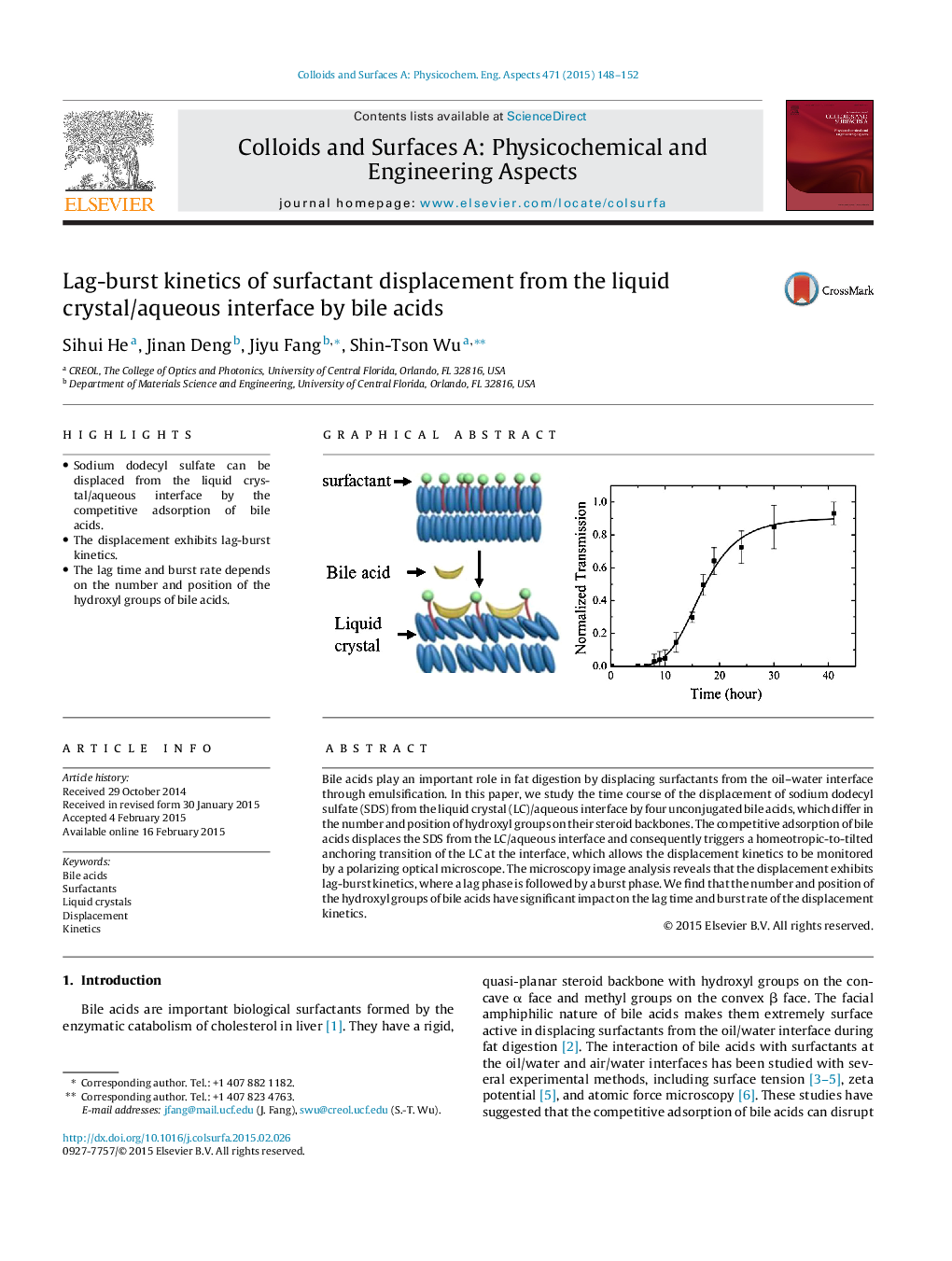
•Sodium dodecyl sulfate can be displaced from the liquid crystal/aqueous interface by the competitive adsorption of bile acids.•The displacement exhibits lag-burst kinetics.•The lag time and burst rate depends on the number and position of the hydroxyl groups of bile acids.
Bile acids play an important role in fat digestion by displacing surfactants from the oil–water interface through emulsification. In this paper, we study the time course of the displacement of sodium dodecyl sulfate (SDS) from the liquid crystal (LC)/aqueous interface by four unconjugated bile acids, which differ in the number and position of hydroxyl groups on their steroid backbones. The competitive adsorption of bile acids displaces the SDS from the LC/aqueous interface and consequently triggers a homeotropic-to-tilted anchoring transition of the LC at the interface, which allows the displacement kinetics to be monitored by a polarizing optical microscope. The microscopy image analysis reveals that the displacement exhibits lag-burst kinetics, where a lag phase is followed by a burst phase. We find that the number and position of the hydroxyl groups of bile acids have significant impact on the lag time and burst rate of the displacement kinetics.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide