| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592366 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2015 | 6 Pages |
•Shake gels can be formed with particles with different shapes or sizes.•Dispersions of poly(ethylene oxide) and laponite, montmorillonite or silica form shake gels.•The shake gel formation depends on the nature of the applied shear field.•The degree of particle coverage necessary for shake gel formation depends on particle shape.
Some dispersions of clay and silica particles in water in the presence of relatively high molecular weight polyethyleneoxide (PEO), which are fluid when at rest, become solid-like after a quick shake to such an extent that they can be held in the hand. On leaving the dispersions for a certain period of time, minutes to days depending on the polymer molecular weight and concentration, the dispersions become liquid-like again. These dispersions have been called “shake gels”, and a number of physical variables are determinant for producing the gel and controlling its behavior. In this work, we have studied the effect of the shape and size of the particles and of the PEO molecular weights. To that aim, we have mapped the “phase” behavior of silica (Ludox TM-50), montmorillonite and laponite dispersions in presence of PEO of different molecular weight. Shake gels are formed under certain concentrations of particles and PEO. The necessary degree of particle coverage for shake gel formation seems to depend on the particle shape. Whereas in the case of disc-shaped particles this limit is around the saturation concentration, in the case of spherical particles the limit is around 2/3 of the particle surface saturation. On the other hand, we observe some differences between montmorillonite (micrometer-size particles) and laponite (nanometric particles) dispersions. When we shake the former we find in some cases an important and irreversible phase separation; on the contrary, in the case of the laponite dispersions, the phase separation is far less frequent and extensive. Finally, we have found that the nature of the applied shear field has a profound effect on the sample behavior. When we place the dispersions in a conventional rheometer and shear them at moderate shear rate, for many minutes the gel is not formed. However, shaking it by hand or extruding it through a syringe brings about this effect in a matter of seconds.
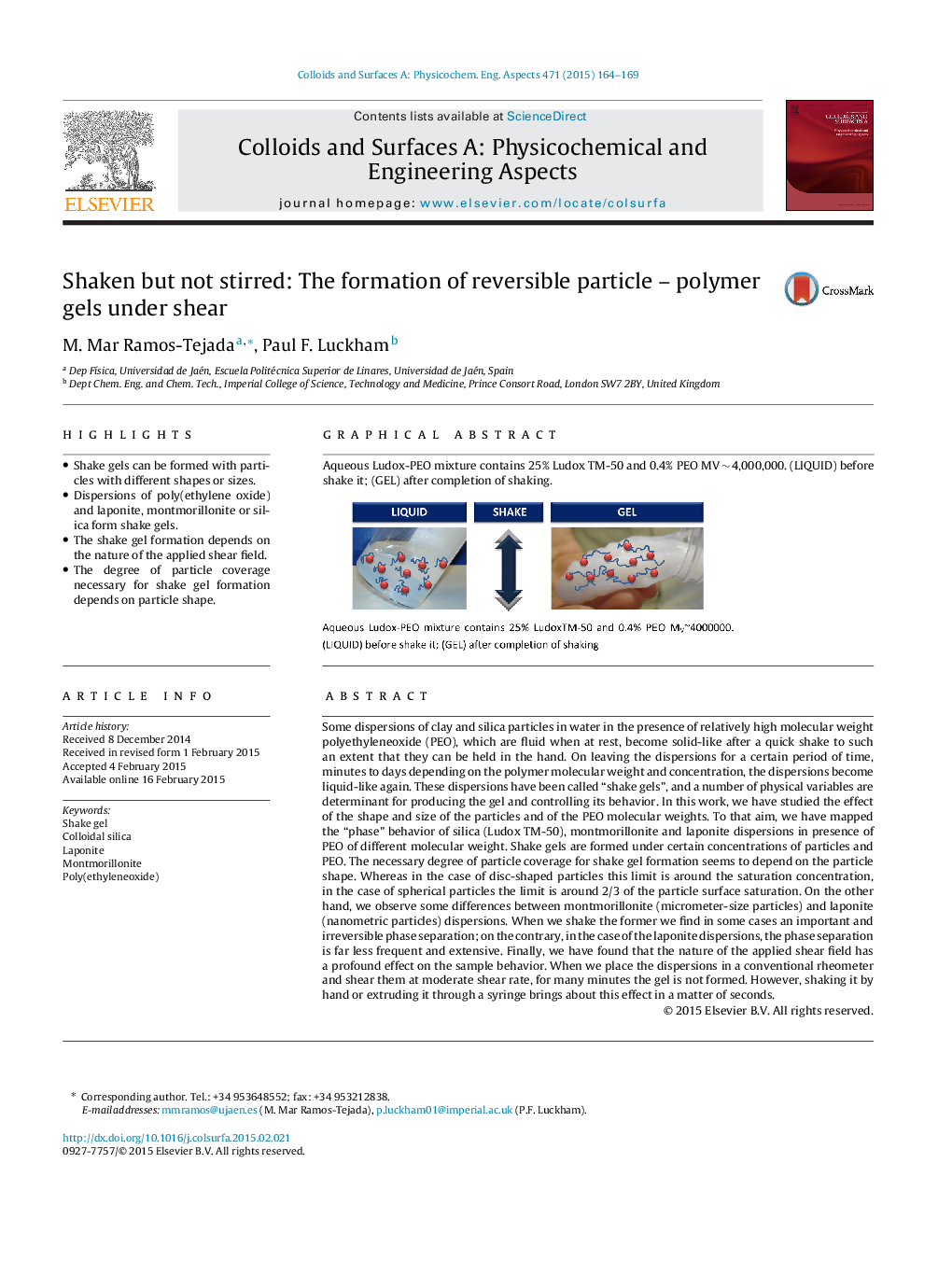
Graphical abstractAqueous Ludox-PEO mixture contains 25% Ludox TM-50 and 0.4% PEO MV ∼ 4,000,000. (LIQUID) before shake it; (GEL) after completion of shaking.Figure optionsDownload full-size imageDownload as PowerPoint slide