| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592390 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2015 | 7 Pages |
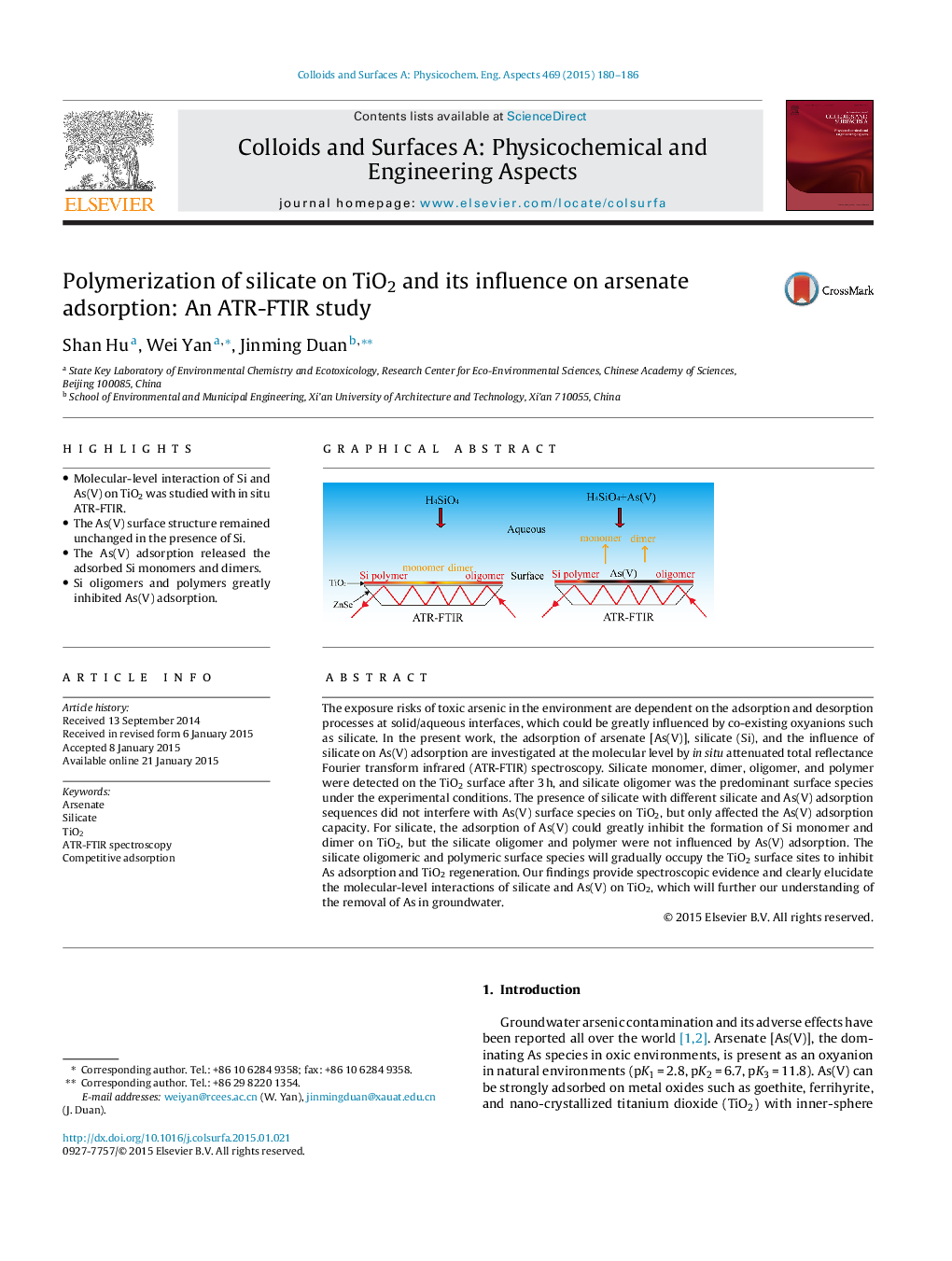
•Molecular-level interaction of Si and As(V) on TiO2 was studied with in situ ATR-FTIR.•The As(V) surface structure remained unchanged in the presence of Si.•The As(V) adsorption released the adsorbed Si monomers and dimers.•Si oligomers and polymers greatly inhibited As(V) adsorption.
The exposure risks of toxic arsenic in the environment are dependent on the adsorption and desorption processes at solid/aqueous interfaces, which could be greatly influenced by co-existing oxyanions such as silicate. In the present work, the adsorption of arsenate [As(V)], silicate (Si), and the influence of silicate on As(V) adsorption are investigated at the molecular level by in situ attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy. Silicate monomer, dimer, oligomer, and polymer were detected on the TiO2 surface after 3 h, and silicate oligomer was the predominant surface species under the experimental conditions. The presence of silicate with different silicate and As(V) adsorption sequences did not interfere with As(V) surface species on TiO2, but only affected the As(V) adsorption capacity. For silicate, the adsorption of As(V) could greatly inhibit the formation of Si monomer and dimer on TiO2, but the silicate oligomer and polymer were not influenced by As(V) adsorption. The silicate oligomeric and polymeric surface species will gradually occupy the TiO2 surface sites to inhibit As adsorption and TiO2 regeneration. Our findings provide spectroscopic evidence and clearly elucidate the molecular-level interactions of silicate and As(V) on TiO2, which will further our understanding of the removal of As in groundwater.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide