| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592581 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2014 | 8 Pages |
•Interactions of surfactants with BSA were investigated by various techniques.•Property of the spacer in Gemini surfactant played an important role.•Gemini surfactants can interact much stronger than single-chain surfactants.
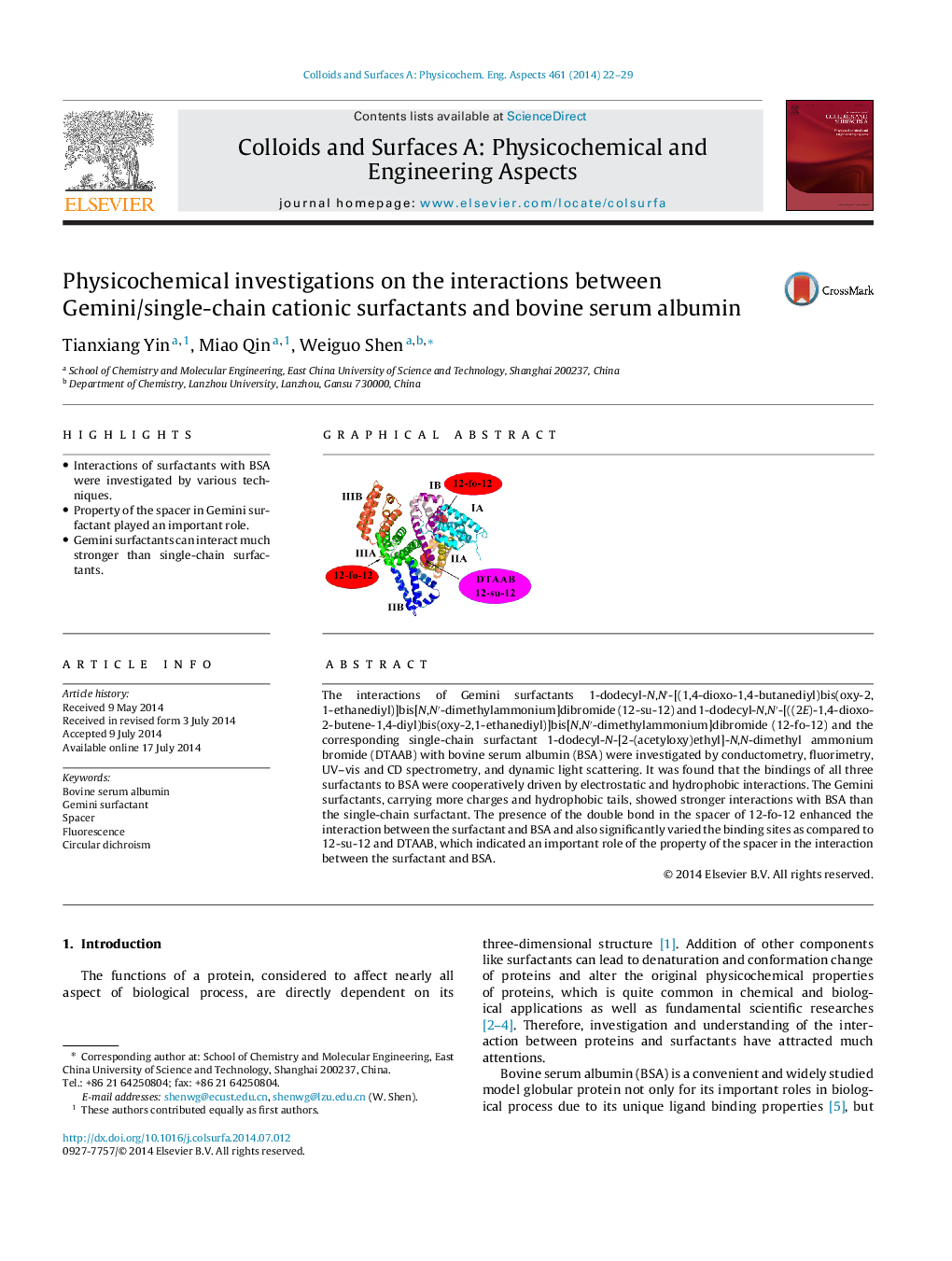
The interactions of Gemini surfactants 1-dodecyl-N,N′-[(1,4-dioxo-1,4-butanediyl)bis(oxy-2,1-ethanediyl)]bis[N,N′-dimethylammonium]dibromide (12-su-12) and 1-dodecyl-N,N′-[((2E)-1,4-dioxo-2-butene-1,4-diyl)bis(oxy-2,1-ethanediyl)]bis[N,N′-dimethylammonium]dibromide (12-fo-12) and the corresponding single-chain surfactant 1-dodecyl-N-[2-(acetyloxy)ethyl]-N,N-dimethyl ammonium bromide (DTAAB) with bovine serum albumin (BSA) were investigated by conductometry, fluorimetry, UV–vis and CD spectrometry, and dynamic light scattering. It was found that the bindings of all three surfactants to BSA were cooperatively driven by electrostatic and hydrophobic interactions. The Gemini surfactants, carrying more charges and hydrophobic tails, showed stronger interactions with BSA than the single-chain surfactant. The presence of the double bond in the spacer of 12-fo-12 enhanced the interaction between the surfactant and BSA and also significantly varied the binding sites as compared to 12-su-12 and DTAAB, which indicated an important role of the property of the spacer in the interaction between the surfactant and BSA.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide