| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592594 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2014 | 7 Pages |
•ZnO NPs undergo chemical transformation in the presence of insoluble phosphate.•HAP induced the transformation of ZnO NPs to scholzite under acid condition.•ZnO NPs transformed to amorphous phase under neutral and basic conditions.
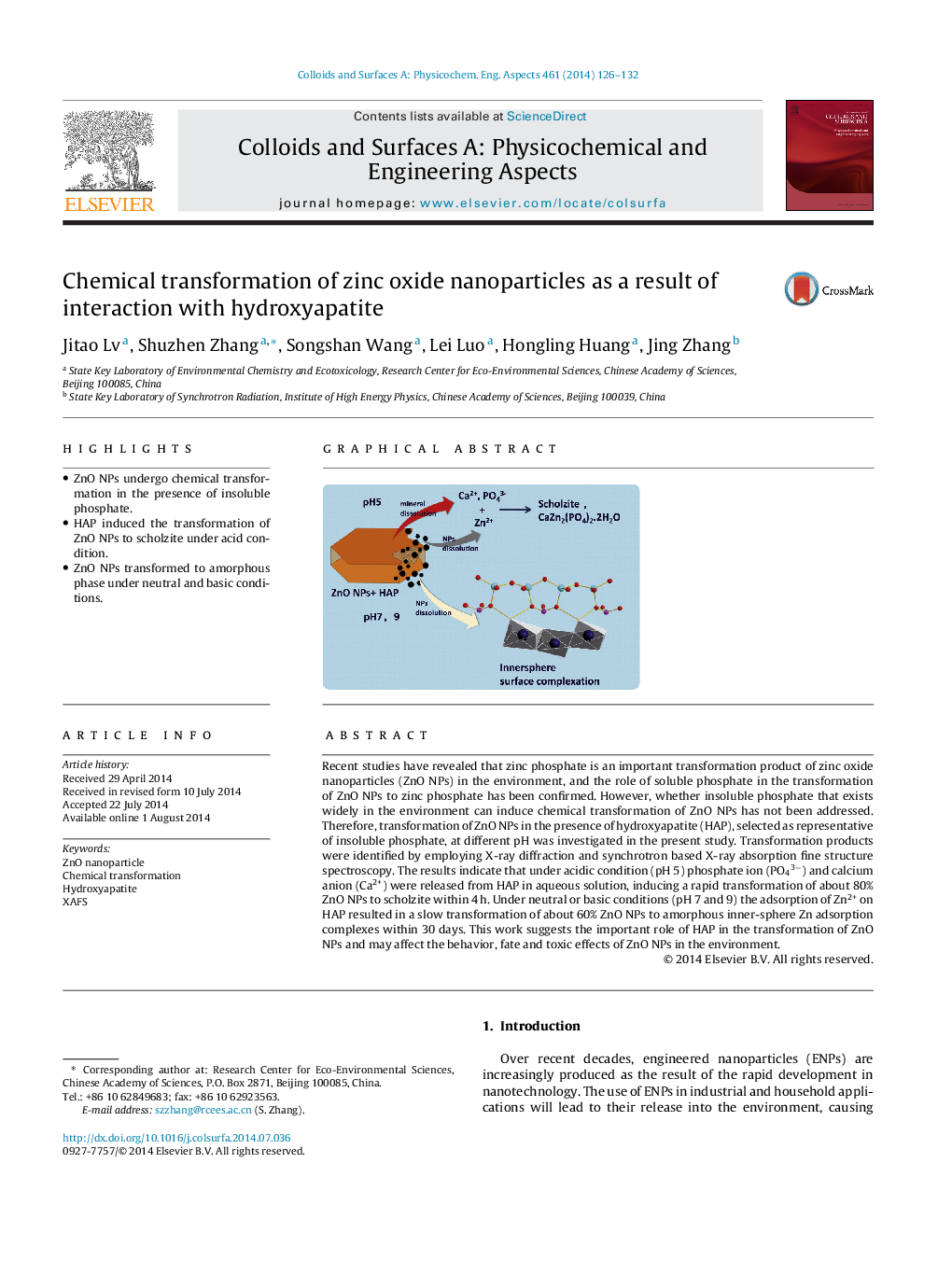
Recent studies have revealed that zinc phosphate is an important transformation product of zinc oxide nanoparticles (ZnO NPs) in the environment, and the role of soluble phosphate in the transformation of ZnO NPs to zinc phosphate has been confirmed. However, whether insoluble phosphate that exists widely in the environment can induce chemical transformation of ZnO NPs has not been addressed. Therefore, transformation of ZnO NPs in the presence of hydroxyapatite (HAP), selected as representative of insoluble phosphate, at different pH was investigated in the present study. Transformation products were identified by employing X-ray diffraction and synchrotron based X-ray absorption fine structure spectroscopy. The results indicate that under acidic condition (pH 5) phosphate ion (PO43−) and calcium anion (Ca2+) were released from HAP in aqueous solution, inducing a rapid transformation of about 80% ZnO NPs to scholzite within 4 h. Under neutral or basic conditions (pH 7 and 9) the adsorption of Zn2+ on HAP resulted in a slow transformation of about 60% ZnO NPs to amorphous inner-sphere Zn adsorption complexes within 30 days. This work suggests the important role of HAP in the transformation of ZnO NPs and may affect the behavior, fate and toxic effects of ZnO NPs in the environment.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide