| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592623 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2014 | 8 Pages |
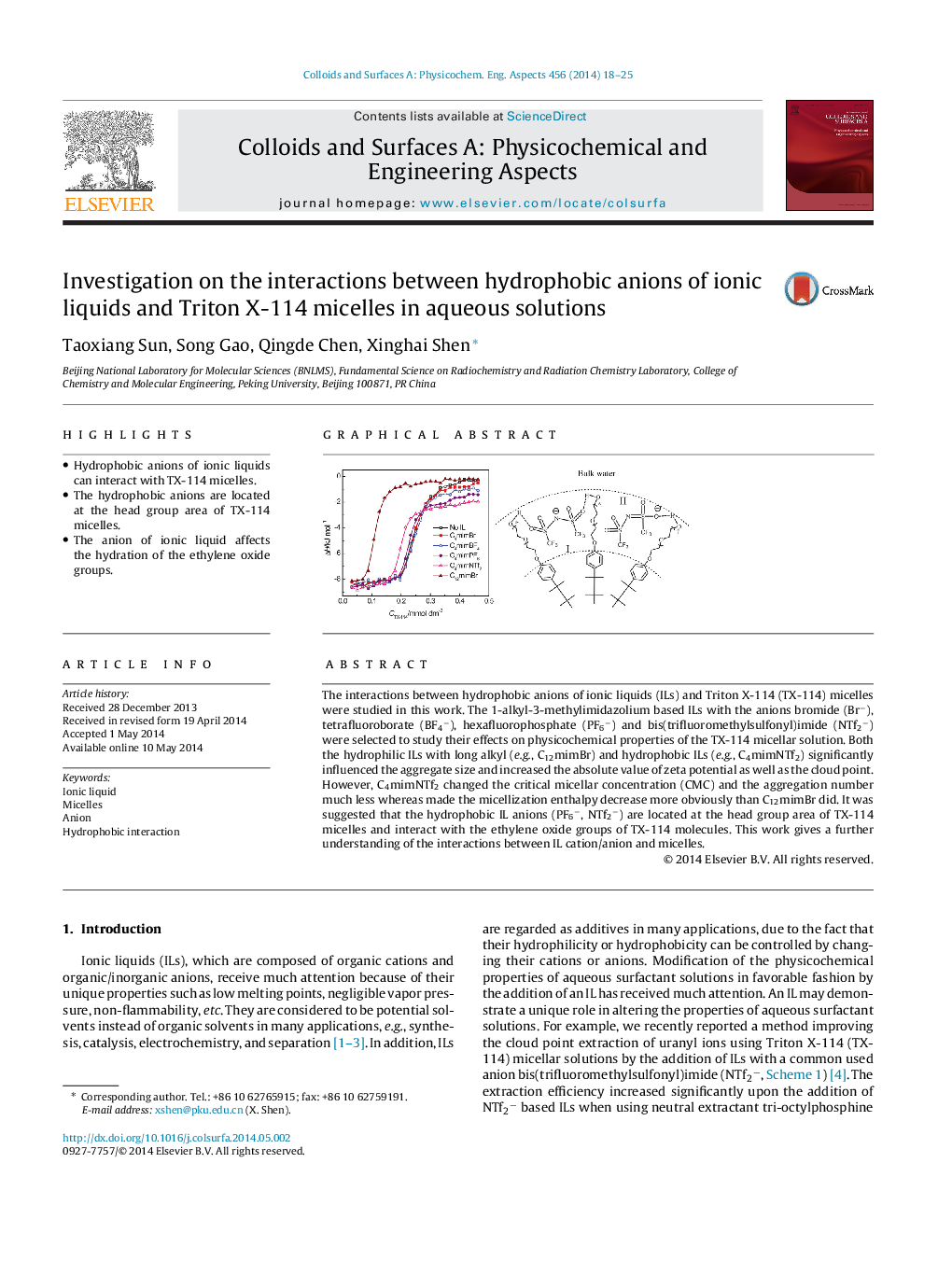
•Hydrophobic anions of ionic liquids can interact with TX-114 micelles.•The hydrophobic anions are located at the head group area of TX-114 micelles.•The anion of ionic liquid affects the hydration of the ethylene oxide groups.
The interactions between hydrophobic anions of ionic liquids (ILs) and Triton X-114 (TX-114) micelles were studied in this work. The 1-alkyl-3-methylimidazolium based ILs with the anions bromide (Br−), tetrafluoroborate (BF4−), hexafluorophosphate (PF6−) and bis(trifluoromethylsulfonyl)imide (NTf2−) were selected to study their effects on physicochemical properties of the TX-114 micellar solution. Both the hydrophilic ILs with long alkyl (e.g., C12mimBr) and hydrophobic ILs (e.g., C4mimNTf2) significantly influenced the aggregate size and increased the absolute value of zeta potential as well as the cloud point. However, C4mimNTf2 changed the critical micellar concentration (CMC) and the aggregation number much less whereas made the micellization enthalpy decrease more obviously than C12mimBr did. It was suggested that the hydrophobic IL anions (PF6−, NTf2−) are located at the head group area of TX-114 micelles and interact with the ethylene oxide groups of TX-114 molecules. This work gives a further understanding of the interactions between IL cation/anion and micelles.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide